Cubic Equations of State (thermo.eos)¶
This module contains implementations of most cubic equations of state for pure components. This includes Peng-Robinson, SRK, Van der Waals, PRSV, TWU and many other variants.
For reporting bugs, adding feature requests, or submitting pull requests, please use the GitHub issue tracker.
Base Class¶
- class thermo.eos.GCEOS[source]¶
Bases:
objectClass for solving a generic Pressure-explicit three-parameter cubic equation of state. Does not implement any parameters itself; must be subclassed by an equation of state class which uses it. Works for mixtures or pure species for all properties except fugacity. All properties are derived with the CAS SymPy, not relying on any derivations previously published.
The main methods (in order they are called) are
GCEOS.solve,GCEOS.set_from_PT,GCEOS.volume_solutions, andGCEOS.set_properties_from_solution.GCEOS.solvecallsGCEOS.check_sufficient_inputs, which checks if two of T, P, and V were set. It then solves for the remaining variable. If T is missing, methodGCEOS.solve_Tis used; it is parameter specific, and so must be implemented in each specific EOS. If P is missing, it is directly calculated. If V is missing, it is calculated with the methodGCEOS.volume_solutions. At this point, either three possible volumes or one user specified volume are known. The value of a_alpha, and its first and second temperature derivative are calculated with the EOS-specific methodGCEOS.a_alpha_and_derivatives.If V is not provided,
GCEOS.volume_solutionscalculates the three possible molar volumes which are solutions to the EOS; in the single-phase region, only one solution is real and correct. In the two-phase region, all volumes are real, but only the largest and smallest solution are physically meaningful, with the largest being that of the gas and the smallest that of the liquid.GCEOS.set_from_PTis called to sort out the possible molar volumes. For the case of a user-specified V, the possibility of there existing another solution is ignored for speed. If there is only one real volume, the methodGCEOS.set_properties_from_solutionis called with it. If there are two real volumes,GCEOS.set_properties_from_solutionis called once with each volume. The phase is returned byGCEOS.set_properties_from_solution, and the volumes is set to eitherGCEOS.V_lorGCEOS.V_gas appropriate.GCEOS.set_properties_from_solutionis a large function which calculates all relevant partial derivatives and properties of the EOS. 17 derivatives and excess enthalpy and entropy are calculated first. Finally, it sets all these properties as attibutes for either the liquid or gas phase with the convention of adding on _l or _g to the variable names, respectively.- Attributes
- T
float Temperature of cubic EOS state, [K]
- P
float Pressure of cubic EOS state, [Pa]
- a
float a parameter of cubic EOS; formulas vary with the EOS, [Pa*m^6/mol^2]
- b
float b parameter of cubic EOS; formulas vary with the EOS, [m^3/mol]
- delta
float Coefficient calculated by EOS-specific method, [m^3/mol]
- epsilon
float Coefficient calculated by EOS-specific method, [m^6/mol^2]
- a_alpha
float Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- da_alpha_dT
float Temperature derivative of calculated by EOS-specific method, [J^2/mol^2/Pa/K]
- d2a_alpha_dT2
float Second temperature derivative of calculated by EOS-specific method, [J^2/mol^2/Pa/K**2]
- Zc
float Critical compressibility of cubic EOS state, [-]
- phase
str One of ‘l’, ‘g’, or ‘l/g’ to represent whether or not there is a liquid-like solution, vapor-like solution, or both available, [-]
- raw_volumes
list[(float,complex), 3] Calculated molar volumes from the volume solver; depending on the state and selected volume solver, imaginary volumes may be represented by 0 or -1j to save the time of actually calculating them, [m^3/mol]
- V_l
float Liquid phase molar volume, [m^3/mol]
- V_g
float Vapor phase molar volume, [m^3/mol]
- V
floatorNone Molar volume specified as input; otherwise None, [m^3/mol]
- Z_l
float Liquid phase compressibility, [-]
- Z_g
float Vapor phase compressibility, [-]
- PIP_l
float Liquid phase phase identification parameter, [-]
- PIP_g
float Vapor phase phase identification parameter, [-]
- dP_dT_l
float Liquid phase temperature derivative of pressure at constant volume, [Pa/K].
- dP_dT_g
float Vapor phase temperature derivative of pressure at constant volume, [Pa/K].
- dP_dV_l
float Liquid phase volume derivative of pressure at constant temperature, [Pa*mol/m^3].
- dP_dV_g
float Gas phase volume derivative of pressure at constant temperature, [Pa*mol/m^3].
- dV_dT_l
float Liquid phase temperature derivative of volume at constant pressure, [m^3/(mol*K)].
- dV_dT_g
float Gas phase temperature derivative of volume at constant pressure, [m^3/(mol*K)].
- dV_dP_l
float Liquid phase pressure derivative of volume at constant temperature, [m^3/(mol*Pa)].
- dV_dP_g
float Gas phase pressure derivative of volume at constant temperature, [m^3/(mol*Pa)].
- dT_dV_l
float Liquid phase volume derivative of temperature at constant pressure, [K*mol/m^3].
- dT_dV_g
float Gas phase volume derivative of temperature at constant pressure, [K*mol/m^3]. See
GCEOS.set_properties_from_solutionfor the formula.- dT_dP_l
float Liquid phase pressure derivative of temperature at constant volume, [K/Pa].
- dT_dP_g
float Gas phase pressure derivative of temperature at constant volume, [K/Pa].
- d2P_dT2_l
float Liquid phase second derivative of pressure with respect to temperature at constant volume, [Pa/K^2].
- d2P_dT2_g
float Gas phase second derivative of pressure with respect to temperature at constant volume, [Pa/K^2].
- d2P_dV2_l
float Liquid phase second derivative of pressure with respect to volume at constant temperature, [Pa*mol^2/m^6].
- d2P_dTdV_l
float Liquid phase second derivative of pressure with respect to volume and then temperature, [Pa*mol/(K*m^3)].
- d2P_dTdV_g
float Gas phase second derivative of pressure with respect to volume and then temperature, [Pa*mol/(K*m^3)].
- H_dep_l
float Liquid phase departure enthalpy, [J/mol]. See
GCEOS.set_properties_from_solutionfor the formula.- H_dep_g
float Gas phase departure enthalpy, [J/mol]. See
GCEOS.set_properties_from_solutionfor the formula.- S_dep_l
float Liquid phase departure entropy, [J/(mol*K)]. See
GCEOS.set_properties_from_solutionfor the formula.- S_dep_g
float Gas phase departure entropy, [J/(mol*K)]. See
GCEOS.set_properties_from_solutionfor the formula.- G_dep_l
float Liquid phase departure Gibbs energy, [J/mol].
- G_dep_g
float Gas phase departure Gibbs energy, [J/mol].
- Cp_dep_l
float Liquid phase departure heat capacity, [J/(mol*K)]
- Cp_dep_g
float Gas phase departure heat capacity, [J/(mol*K)]
- Cv_dep_l
float Liquid phase departure constant volume heat capacity, [J/(mol*K)]. See
GCEOS.set_properties_from_solutionfor the formula.- Cv_dep_g
float Gas phase departure constant volume heat capacity, [J/(mol*K)]. See
GCEOS.set_properties_from_solutionfor the formula.- c1
float Full value of the constant in the a parameter, set in some EOSs, [-]
- c2
float Full value of the constant in the b parameter, set in some EOSs, [-]
A_dep_gDeparture molar Helmholtz energy from ideal gas behavior for the gas phase, [J/mol].
A_dep_lDeparture molar Helmholtz energy from ideal gas behavior for the liquid phase, [J/mol].
beta_gIsobaric (constant-pressure) expansion coefficient for the gas phase, [1/K].
beta_lIsobaric (constant-pressure) expansion coefficient for the liquid phase, [1/K].
Cp_minus_Cv_gCp - Cv for the gas phase, [J/mol/K].
Cp_minus_Cv_lCp - Cv for the liquid phase, [J/mol/K].
d2a_alpha_dTdP_g_VDerivative of the temperature derivative of a_alpha with respect to pressure at constant volume (varying T) for the gas phase, [J^2/mol^2/Pa^2/K].
d2a_alpha_dTdP_l_VDerivative of the temperature derivative of a_alpha with respect to pressure at constant volume (varying T) for the liquid phase, [J^2/mol^2/Pa^2/K].
d2H_dep_dT2_gSecond temperature derivative of departure enthalpy with respect to temperature for the gas phase, [(J/mol)/K^2].
d2H_dep_dT2_g_PSecond temperature derivative of departure enthalpy with respect to temperature for the gas phase, [(J/mol)/K^2].
d2H_dep_dT2_g_VSecond temperature derivative of departure enthalpy with respect to temperature at constant volume for the gas phase, [(J/mol)/K^2].
d2H_dep_dT2_lSecond temperature derivative of departure enthalpy with respect to temperature for the liquid phase, [(J/mol)/K^2].
d2H_dep_dT2_l_PSecond temperature derivative of departure enthalpy with respect to temperature for the liquid phase, [(J/mol)/K^2].
d2H_dep_dT2_l_VSecond temperature derivative of departure enthalpy with respect to temperature at constant volume for the liquid phase, [(J/mol)/K^2].
d2H_dep_dTdP_gTemperature and pressure derivative of departure enthalpy at constant pressure then temperature for the gas phase, [(J/mol)/K/Pa].
d2H_dep_dTdP_lTemperature and pressure derivative of departure enthalpy at constant pressure then temperature for the liquid phase, [(J/mol)/K/Pa].
d2P_drho2_gSecond derivative of pressure with respect to molar density for the gas phase, [Pa/(mol/m^3)^2].
d2P_drho2_lSecond derivative of pressure with respect to molar density for the liquid phase, [Pa/(mol/m^3)^2].
d2P_dT2_PV_gSecond derivative of pressure with respect to temperature twice, but with pressure held constant the first time and volume held constant the second time for the gas phase, [Pa/K^2].
d2P_dT2_PV_lSecond derivative of pressure with respect to temperature twice, but with pressure held constant the first time and volume held constant the second time for the liquid phase, [Pa/K^2].
d2P_dTdP_gSecond derivative of pressure with respect to temperature and, then pressure; and with volume held constant at first, then temperature, for the gas phase, [1/K].
d2P_dTdP_lSecond derivative of pressure with respect to temperature and, then pressure; and with volume held constant at first, then temperature, for the liquid phase, [1/K].
d2P_dTdrho_gDerivative of pressure with respect to molar density, and temperature for the gas phase, [Pa/(K*mol/m^3)].
d2P_dTdrho_lDerivative of pressure with respect to molar density, and temperature for the liquid phase, [Pa/(K*mol/m^3)].
d2P_dVdP_gSecond derivative of pressure with respect to molar volume and then pressure for the gas phase, [mol/m^3].
d2P_dVdP_lSecond derivative of pressure with respect to molar volume and then pressure for the liquid phase, [mol/m^3].
d2P_dVdT_gAlias of
GCEOS.d2P_dTdV_gd2P_dVdT_lAlias of
GCEOS.d2P_dTdV_ld2P_dVdT_TP_gSecond derivative of pressure with respect to molar volume and then temperature at constant temperature then pressure for the gas phase, [Pa*mol/m^3/K].
d2P_dVdT_TP_lSecond derivative of pressure with respect to molar volume and then temperature at constant temperature then pressure for the liquid phase, [Pa*mol/m^3/K].
d2rho_dP2_gSecond derivative of molar density with respect to pressure for the gas phase, [(mol/m^3)/Pa^2].
d2rho_dP2_lSecond derivative of molar density with respect to pressure for the liquid phase, [(mol/m^3)/Pa^2].
d2rho_dPdT_gSecond derivative of molar density with respect to pressure and temperature for the gas phase, [(mol/m^3)/(K*Pa)].
d2rho_dPdT_lSecond derivative of molar density with respect to pressure and temperature for the liquid phase, [(mol/m^3)/(K*Pa)].
d2rho_dT2_gSecond derivative of molar density with respect to temperature for the gas phase, [(mol/m^3)/K^2].
d2rho_dT2_lSecond derivative of molar density with respect to temperature for the liquid phase, [(mol/m^3)/K^2].
d2S_dep_dT2_gSecond temperature derivative of departure entropy with respect to temperature for the gas phase, [(J/mol)/K^3].
d2S_dep_dT2_g_VSecond temperature derivative of departure entropy with respect to temperature at constant volume for the gas phase, [(J/mol)/K^3].
d2S_dep_dT2_lSecond temperature derivative of departure entropy with respect to temperature for the liquid phase, [(J/mol)/K^3].
d2S_dep_dT2_l_VSecond temperature derivative of departure entropy with respect to temperature at constant volume for the liquid phase, [(J/mol)/K^3].
d2S_dep_dTdP_gTemperature and pressure derivative of departure entropy at constant pressure then temperature for the gas phase, [(J/mol)/K^2/Pa].
d2S_dep_dTdP_lTemperature and pressure derivative of departure entropy at constant pressure then temperature for the liquid phase, [(J/mol)/K^2/Pa].
d2T_dP2_gSecond partial derivative of temperature with respect to pressure (constant volume) for the gas phase, [K/Pa^2].
d2T_dP2_lSecond partial derivative of temperature with respect to pressure (constant temperature) for the liquid phase, [K/Pa^2].
d2T_dPdrho_gDerivative of temperature with respect to molar density, and pressure for the gas phase, [K/(Pa*mol/m^3)].
d2T_dPdrho_lDerivative of temperature with respect to molar density, and pressure for the liquid phase, [K/(Pa*mol/m^3)].
d2T_dPdV_gSecond partial derivative of temperature with respect to pressure (constant volume) and then volume (constant pressure) for the gas phase, [K*mol/(Pa*m^3)].
d2T_dPdV_lSecond partial derivative of temperature with respect to pressure (constant volume) and then volume (constant pressure) for the liquid phase, [K*mol/(Pa*m^3)].
d2T_drho2_gSecond derivative of temperature with respect to molar density for the gas phase, [K/(mol/m^3)^2].
d2T_drho2_lSecond derivative of temperature with respect to molar density for the liquid phase, [K/(mol/m^3)^2].
d2T_dV2_gSecond partial derivative of temperature with respect to volume (constant pressure) for the gas phase, [K*mol^2/m^6].
d2T_dV2_lSecond partial derivative of temperature with respect to volume (constant pressure) for the liquid phase, [K*mol^2/m^6].
d2T_dVdP_gSecond partial derivative of temperature with respect to pressure (constant volume) and then volume (constant pressure) for the gas phase, [K*mol/(Pa*m^3)].
d2T_dVdP_lSecond partial derivative of temperature with respect to pressure (constant volume) and then volume (constant pressure) for the liquid phase, [K*mol/(Pa*m^3)].
d2V_dP2_gSecond partial derivative of volume with respect to pressure (constant temperature) for the gas phase, [m^3/(Pa^2*mol)].
d2V_dP2_lSecond partial derivative of volume with respect to pressure (constant temperature) for the liquid phase, [m^3/(Pa^2*mol)].
d2V_dPdT_gSecond partial derivative of volume with respect to pressure (constant temperature) and then presssure (constant temperature) for the gas phase, [m^3/(K*Pa*mol)].
d2V_dPdT_lSecond partial derivative of volume with respect to pressure (constant temperature) and then presssure (constant temperature) for the liquid phase, [m^3/(K*Pa*mol)].
d2V_dT2_gSecond partial derivative of volume with respect to temperature (constant pressure) for the gas phase, [m^3/(mol*K^2)].
d2V_dT2_lSecond partial derivative of volume with respect to temperature (constant pressure) for the liquid phase, [m^3/(mol*K^2)].
d2V_dTdP_gSecond partial derivative of volume with respect to pressure (constant temperature) and then presssure (constant temperature) for the gas phase, [m^3/(K*Pa*mol)].
d2V_dTdP_lSecond partial derivative of volume with respect to pressure (constant temperature) and then presssure (constant temperature) for the liquid phase, [m^3/(K*Pa*mol)].
d3a_alpha_dT3Method to calculate the third temperature derivative of , [J^2/mol^2/Pa/K^3].
da_alpha_dP_g_VDerivative of the a_alpha with respect to pressure at constant volume (varying T) for the gas phase, [J^2/mol^2/Pa^2].
da_alpha_dP_l_VDerivative of the a_alpha with respect to pressure at constant volume (varying T) for the liquid phase, [J^2/mol^2/Pa^2].
dbeta_dP_gDerivative of isobaric expansion coefficient with respect to pressure for the gas phase, [1/(Pa*K)].
dbeta_dP_lDerivative of isobaric expansion coefficient with respect to pressure for the liquid phase, [1/(Pa*K)].
dbeta_dT_gDerivative of isobaric expansion coefficient with respect to temperature for the gas phase, [1/K^2].
dbeta_dT_lDerivative of isobaric expansion coefficient with respect to temperature for the liquid phase, [1/K^2].
dfugacity_dP_gDerivative of fugacity with respect to pressure for the gas phase, [-].
dfugacity_dP_lDerivative of fugacity with respect to pressure for the liquid phase, [-].
dfugacity_dT_gDerivative of fugacity with respect to temperature for the gas phase, [Pa/K].
dfugacity_dT_lDerivative of fugacity with respect to temperature for the liquid phase, [Pa/K].
dH_dep_dP_gDerivative of departure enthalpy with respect to pressure for the gas phase, [(J/mol)/Pa].
dH_dep_dP_g_VDerivative of departure enthalpy with respect to pressure at constant volume for the liquid phase, [(J/mol)/Pa].
dH_dep_dP_lDerivative of departure enthalpy with respect to pressure for the liquid phase, [(J/mol)/Pa].
dH_dep_dP_l_VDerivative of departure enthalpy with respect to pressure at constant volume for the gas phase, [(J/mol)/Pa].
dH_dep_dT_gDerivative of departure enthalpy with respect to temperature for the gas phase, [(J/mol)/K].
dH_dep_dT_g_VDerivative of departure enthalpy with respect to temperature at constant volume for the gas phase, [(J/mol)/K].
dH_dep_dT_lDerivative of departure enthalpy with respect to temperature for the liquid phase, [(J/mol)/K].
dH_dep_dT_l_VDerivative of departure enthalpy with respect to temperature at constant volume for the liquid phase, [(J/mol)/K].
dH_dep_dV_g_PDerivative of departure enthalpy with respect to volume at constant pressure for the gas phase, [J/m^3].
dH_dep_dV_g_TDerivative of departure enthalpy with respect to volume at constant temperature for the gas phase, [J/m^3].
dH_dep_dV_l_PDerivative of departure enthalpy with respect to volume at constant pressure for the liquid phase, [J/m^3].
dH_dep_dV_l_TDerivative of departure enthalpy with respect to volume at constant temperature for the gas phase, [J/m^3].
dP_drho_gDerivative of pressure with respect to molar density for the gas phase, [Pa/(mol/m^3)].
dP_drho_lDerivative of pressure with respect to molar density for the liquid phase, [Pa/(mol/m^3)].
dphi_dP_gDerivative of fugacity coefficient with respect to pressure for the gas phase, [1/Pa].
dphi_dP_lDerivative of fugacity coefficient with respect to pressure for the liquid phase, [1/Pa].
dphi_dT_gDerivative of fugacity coefficient with respect to temperature for the gas phase, [1/K].
dphi_dT_lDerivative of fugacity coefficient with respect to temperature for the liquid phase, [1/K].
drho_dP_gDerivative of molar density with respect to pressure for the gas phase, [(mol/m^3)/Pa].
drho_dP_lDerivative of molar density with respect to pressure for the liquid phase, [(mol/m^3)/Pa].
drho_dT_gDerivative of molar density with respect to temperature for the gas phase, [(mol/m^3)/K].
drho_dT_lDerivative of molar density with respect to temperature for the liquid phase, [(mol/m^3)/K].
dS_dep_dP_gDerivative of departure entropy with respect to pressure for the gas phase, [(J/mol)/K/Pa].
dS_dep_dP_g_VDerivative of departure entropy with respect to pressure at constant volume for the gas phase, [(J/mol)/K/Pa].
dS_dep_dP_lDerivative of departure entropy with respect to pressure for the liquid phase, [(J/mol)/K/Pa].
dS_dep_dP_l_VDerivative of departure entropy with respect to pressure at constant volume for the liquid phase, [(J/mol)/K/Pa].
dS_dep_dT_gDerivative of departure entropy with respect to temperature for the gas phase, [(J/mol)/K^2].
dS_dep_dT_g_VDerivative of departure entropy with respect to temperature at constant volume for the gas phase, [(J/mol)/K^2].
dS_dep_dT_lDerivative of departure entropy with respect to temperature for the liquid phase, [(J/mol)/K^2].
dS_dep_dT_l_VDerivative of departure entropy with respect to temperature at constant volume for the liquid phase, [(J/mol)/K^2].
dS_dep_dV_g_PDerivative of departure entropy with respect to volume at constant pressure for the gas phase, [J/K/m^3].
dS_dep_dV_g_TDerivative of departure entropy with respect to volume at constant temperature for the gas phase, [J/K/m^3].
dS_dep_dV_l_PDerivative of departure entropy with respect to volume at constant pressure for the liquid phase, [J/K/m^3].
dS_dep_dV_l_TDerivative of departure entropy with respect to volume at constant temperature for the gas phase, [J/K/m^3].
dT_drho_gDerivative of temperature with respect to molar density for the gas phase, [K/(mol/m^3)].
dT_drho_lDerivative of temperature with respect to molar density for the liquid phase, [K/(mol/m^3)].
dZ_dP_gDerivative of compressibility factor with respect to pressure for the gas phase, [1/Pa].
dZ_dP_lDerivative of compressibility factor with respect to pressure for the liquid phase, [1/Pa].
dZ_dT_gDerivative of compressibility factor with respect to temperature for the gas phase, [1/K].
dZ_dT_lDerivative of compressibility factor with respect to temperature for the liquid phase, [1/K].
fugacity_gFugacity for the gas phase, [Pa].
fugacity_lFugacity for the liquid phase, [Pa].
kappa_gIsothermal (constant-temperature) expansion coefficient for the gas phase, [1/Pa].
kappa_lIsothermal (constant-temperature) expansion coefficient for the liquid phase, [1/Pa].
lnphi_gThe natural logarithm of the fugacity coefficient for the gas phase, [-].
lnphi_lThe natural logarithm of the fugacity coefficient for the liquid phase, [-].
more_stable_phaseChecks the Gibbs energy of each possible phase, and returns ‘l’ if the liquid-like phase is more stable, and ‘g’ if the vapor-like phase is more stable.
mpmath_volume_ratiosMethod to compare, as ratios, the volumes of the implemented cubic solver versus those calculated using mpmath.
mpmath_volumesMethod to calculate to a high precision the exact roots to the cubic equation, using mpmath.
mpmath_volumes_floatMethod to calculate real roots of a cubic equation, using mpmath, but returned as floats.
phi_gFugacity coefficient for the gas phase, [Pa].
phi_lFugacity coefficient for the liquid phase, [Pa].
rho_gGas molar density, [mol/m^3].
rho_lLiquid molar density, [mol/m^3].
sorted_volumesList of lexicographically-sorted molar volumes available from the root finding algorithm used to solve the PT point.
state_specsConvenience method to return the two specified state specs (T, P, or V) as a dictionary.
U_dep_gDeparture molar internal energy from ideal gas behavior for the gas phase, [J/mol].
U_dep_lDeparture molar internal energy from ideal gas behavior for the liquid phase, [J/mol].
VcCritical volume, [m^3/mol].
V_dep_gDeparture molar volume from ideal gas behavior for the gas phase, [m^3/mol].
V_dep_lDeparture molar volume from ideal gas behavior for the liquid phase, [m^3/mol].
V_g_mpmathThe molar volume of the gas phase calculated with mpmath to a higher precision, [m^3/mol].
V_l_mpmathThe molar volume of the liquid phase calculated with mpmath to a higher precision, [m^3/mol].
- T
Methods
Hvap(T)Method to calculate enthalpy of vaporization for a pure fluid from an equation of state, without iteration.
PT_surface_special([Tmin, Tmax, Pmin, Pmax, ...])Method to create a plot of the special curves of a pure fluid - vapor pressure, determinant zeros, pseudo critical point, and mechanical critical point.
P_PIP_transition(T[, low_P_limit])Method to calculate the pressure which makes the phase identification parameter exactly 1.
Method to calculate the pressure which zero the discriminant function of the general cubic eos, and is likely to sit on a boundary between not having a vapor-like volume; and having a vapor-like volume.
Method to calculate the pressure which zero the discriminant function of the general cubic eos, and is likely to sit on a boundary between not having a liquid-like volume; and having a liquid-like volume.
Method to calculate the pressures which zero the discriminant function of the general cubic eos, at the current temperature.
P_discriminant_zeros_analytical(T, b, delta, ...)Method to calculate the pressures which zero the discriminant function of the general cubic eos.
P_max_at_V(V)Dummy method.
Psat(T[, polish, guess])Generic method to calculate vapor pressure for a specified T.
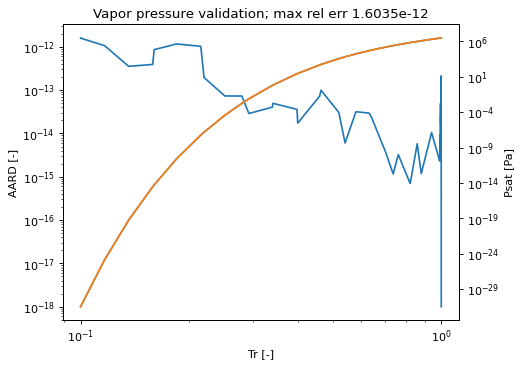
Psat_errors([Tmin, Tmax, pts, plot, show, ...])Method to create a plot of vapor pressure and the relative error of its calculation vs.
T_discriminant_zero_g([T_guess])Method to calculate the temperature which zeros the discriminant function of the general cubic eos, and is likely to sit on a boundary between not having a vapor-like volume; and having a vapor-like volume.
T_discriminant_zero_l([T_guess])Method to calculate the temperature which zeros the discriminant function of the general cubic eos, and is likely to sit on a boundary between not having a liquid-like volume; and having a liquid-like volume.
T_max_at_V(V[, Pmax])Method to calculate the maximum temperature the EOS can create at a constant volume, if one exists; returns None otherwise.
T_min_at_V(V[, Pmin])Returns the minimum temperature for the EOS to have the volume as specified.
Tsat(P[, polish])Generic method to calculate the temperature for a specified vapor pressure of the pure fluid.
V_g_sat(T)Method to calculate molar volume of the vapor phase along the saturation line.
V_l_sat(T)Method to calculate molar volume of the liquid phase along the saturation line.
Method to calculate real roots of a cubic equation, using mpmath.
a_alpha_and_derivatives(T[, full, quick, ...])Method to calculate and its first and second derivatives.
Dummy method to calculate and its first and second derivatives.
a_alpha_for_Psat(T, Psat[, a_alpha_guess])Method to calculate which value of is required for a given T, Psat pair.
a_alpha_for_V(T, P, V)Method to calculate which value of is required for a given T, P pair to match a specified V.
a_alpha_plot([Tmin, Tmax, pts, plot, show])Method to create a plot of the parameter and its first two derivatives.
as_json()Method to create a JSON-friendly serialization of the eos which can be stored, and reloaded later.
Method to an exception if none of the pairs (T, P), (T, V), or (P, V) are given.
d2phi_sat_dT2(T[, polish])Method to calculate the second temperature derivative of saturation fugacity coefficient of the compound.
dH_dep_dT_sat_g(T[, polish])Method to calculate and return the temperature derivative of saturation vapor excess enthalpy.
dH_dep_dT_sat_l(T[, polish])Method to calculate and return the temperature derivative of saturation liquid excess enthalpy.
dPsat_dT(T[, polish, also_Psat])Generic method to calculate the temperature derivative of vapor pressure for a specified T.
dS_dep_dT_sat_g(T[, polish])Method to calculate and return the temperature derivative of saturation vapor excess entropy.
dS_dep_dT_sat_l(T[, polish])Method to calculate and return the temperature derivative of saturation liquid excess entropy.
discriminant([T, P])Method to compute the discriminant of the cubic volume solution with the current EOS parameters, optionally at the same (assumed) T, and P or at different ones, if values are specified.
dphi_sat_dT(T[, polish])Method to calculate the temperature derivative of saturation fugacity coefficient of the compound.
from_json(json_repr)Method to create a eos from a JSON serialization of another eos.
Basic method to calculate a hash of the non-state parts of the model This is useful for comparing to models to determine if they are the same, i.e. in a VLL flash it is important to know if both liquids have the same model.
phi_sat(T[, polish])Method to calculate the saturation fugacity coefficient of the compound.
Generic method to resolve the eos with fully calculated alpha derviatives.
saturation_prop_plot(prop[, Tmin, Tmax, ...])Method to create a plot of a specified property of the EOS along the (pure component) saturation line.
set_from_PT(Vs[, only_l, only_g])Counts the number of real volumes in Vs, and determines what to do.
set_properties_from_solution(T, P, V, b, ...)Sets all interesting properties which can be calculated from an EOS alone.
solve([pure_a_alphas, only_l, only_g, ...])First EOS-generic method; should be called by all specific EOSs.
solve_T(P, V[, solution])Generic method to calculate T from a specified P and V.
Generic method to ensure both volumes, if solutions are physical, have calculated properties.
Basic method to calculate a hash of the state of the model and its model parameters.
to([T, P, V])Method to construct a new EOS object at two of T, P or V.
to_PV(P, V)Method to construct a new EOS object at the spcified P and V.
to_TP(T, P)Method to construct a new EOS object at the spcified T and P.
to_TV(T, V)Method to construct a new EOS object at the spcified T and V.
Method to calculate the relative absolute error in the calculated molar volumes.
volume_errors([Tmin, Tmax, Pmin, Pmax, pts, ...])Method to create a plot of the relative absolute error in the cubic volume solution as compared to a higher-precision calculation.
volume_solutions(T, P, b, delta, epsilon, ...)Halley's method based solver for cubic EOS volumes based on the idea of initializing from a single liquid-like guess which is solved precisely, deflating the cubic analytically, solving the quadratic equation for the next two volumes, and then performing two halley steps on each of them to obtain the final solutions.
volume_solutions_full(T, P, b, delta, ...[, ...])Newton-Raphson based solver for cubic EOS volumes based on the idea of initializing from an analytical solver.
volume_solutions_mp(T, P, b, delta, epsilon, ...)Solution of this form of the cubic EOS in terms of volumes, using the mpmath arbitrary precision library.
- property A_dep_g¶
Departure molar Helmholtz energy from ideal gas behavior for the gas phase, [J/mol].
- property A_dep_l¶
Departure molar Helmholtz energy from ideal gas behavior for the liquid phase, [J/mol].
- property Cp_minus_Cv_g¶
Cp - Cv for the gas phase, [J/mol/K].
- property Cp_minus_Cv_l¶
Cp - Cv for the liquid phase, [J/mol/K].
- Hvap(T)[source]¶
Method to calculate enthalpy of vaporization for a pure fluid from an equation of state, without iteration.
Results above the critical temperature are meaningless. A first-order polynomial is used to extrapolate under 0.32 Tc; however, there is normally not a volume solution to the EOS which can produce that low of a pressure.
- Parameters
- T
float Temperature, [K]
- T
- Returns
- Hvap
float Increase in enthalpy needed for vaporization of liquid phase along the saturation line, [J/mol]
- Hvap
Notes
Calculates vapor pressure and its derivative with Psat and dPsat_dT as well as molar volumes of the saturation liquid and vapor phase in the process.
Very near the critical point this provides unrealistic results due to Psat’s polynomials being insufficiently accurate.
References
- 1
Walas, Stanley M. Phase Equilibria in Chemical Engineering. Butterworth-Heinemann, 1985.
- N = 1¶
The number of components in the EOS
- PT_surface_special(Tmin=0.0001, Tmax=10000.0, Pmin=0.01, Pmax=1000000000.0, pts=50, show=False, color_map=None, mechanical=True, pseudo_critical=True, Psat=True, determinant_zeros=True, phase_ID_transition=True, base_property='V', base_min=None, base_max=None, base_selection='Gmin')[source]¶
Method to create a plot of the special curves of a pure fluid - vapor pressure, determinant zeros, pseudo critical point, and mechanical critical point.
The color background is a plot of the molar volume (by default) which has the minimum Gibbs energy (by default). If shown with a sufficient number of points, the curve between vapor and liquid should be shown smoothly.
When called on a mixture, this method does not have physical significance for the Psat term.
- Parameters
- Tmin
float,optional Minimum temperature of calculation, [K]
- Tmax
float,optional Maximum temperature of calculation, [K]
- Pmin
float,optional Minimum pressure of calculation, [Pa]
- Pmax
float,optional Maximum pressure of calculation, [Pa]
- pts
int,optional The number of points to include in both the x and y axis [-]
- showbool,
optional Whether or not the plot should be rendered and shown; a handle to it is returned if plot is True for other purposes such as saving the plot to a file, [-]
- color_map
matplotlib.cm.ListedColormap,optional Matplotlib colormap object, [-]
- mechanicalbool,
optional Whether or not to include the mechanical critical point; this is the same as the critical point for a pure compound but not for a mixture, [-]
- pseudo_criticalbool,
optional Whether or not to include the pseudo critical point; this is the same as the critical point for a pure compound but not for a mixture, [-]
- Psatbool,
optional Whether or not to include the vapor pressure curve; for mixtures this is neither the bubble nor dew curve, but rather a hypothetical one which uses the same equation as the pure components, [-]
- determinant_zerosbool,
optional Whether or not to include a curve showing when the EOS’s determinant hits zero, [-]
- phase_ID_transitionbool,
optional Whether or not to show a curve of where the PIP hits 1 exactly, [-]
- base_property
str,optional The property which should be plotted; ‘_l’ and ‘_g’ are added automatically according to the selected phase, [-]
- base_min
float,optional If specified, the base property will values will be limited to this value at the minimum, [-]
- base_max
float,optional If specified, the base property will values will be limited to this value at the maximum, [-]
- base_selection
str,optional For the base property, there are often two possible phases and but only one value can be plotted; use ‘l’ to pefer liquid-like values, ‘g’ to prefer gas-like values, and ‘Gmin’ to prefer values of the phase with the lowest Gibbs energy, [-]
- Tmin
- Returns
- fig
matplotlib.figure.Figure Plotted figure, only returned if plot is True, [-]
- fig
- P_PIP_transition(T, low_P_limit=0.0)[source]¶
Method to calculate the pressure which makes the phase identification parameter exactly 1. There are three regions for this calculation:
subcritical - PIP = 1 for the gas-like phase at P = 0
initially supercritical - PIP = 1 on a curve starting at the critical point, increasing for a while, decreasing for a while, and then curving sharply back to a zero pressure.
later supercritical - PIP = 1 for the liquid-like phase at P = 0
- Parameters
- Returns
- P
float Pressure which makes the PIP = 1, [Pa]
- P
Notes
The transition between the region where this function returns values and the high temperature region that doesn’t is the Joule-Thomson inversion point at a pressure of zero and can be directly solved for.
Examples
>>> eos = PRTranslatedConsistent(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6) >>> eos.P_PIP_transition(100) 0.0 >>> low_T = eos.to(T=100.0, P=eos.P_PIP_transition(100, low_P_limit=1e-5)) >>> low_T.PIP_l, low_T.PIP_g (45.778088191, 0.9999999997903) >>> initial_super = eos.to(T=600.0, P=eos.P_PIP_transition(600)) >>> initial_super.P, initial_super.PIP_g (6456282.17132, 0.999999999999) >>> high_T = eos.to(T=900.0, P=eos.P_PIP_transition(900, low_P_limit=1e-5)) >>> high_T.P, high_T.PIP_g (12536704.763, 0.9999999999)
- P_discriminant_zero_g()[source]¶
Method to calculate the pressure which zero the discriminant function of the general cubic eos, and is likely to sit on a boundary between not having a vapor-like volume; and having a vapor-like volume.
- Returns
- P_discriminant_zero_g
float Pressure which make the discriminants zero at the right condition, [Pa]
- P_discriminant_zero_g
Examples
>>> eos = PRTranslatedConsistent(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6) >>> P_trans = eos.P_discriminant_zero_g() >>> P_trans 149960391.7
In this case, the discriminant transition does not reveal a transition to two roots being available, only negative roots becoming negative and imaginary.
>>> eos.to(T=eos.T, P=P_trans*.99999999).mpmath_volumes_float ((-0.0001037013146195082-1.5043987866732543e-08j), (-0.0001037013146195082+1.5043987866732543e-08j), (0.00011799201928619508+0j)) >>> eos.to(T=eos.T, P=P_trans*1.0000001).mpmath_volumes_float ((-0.00010374888853182635+0j), (-0.00010365374200380354+0j), (0.00011799201875924273+0j))
- P_discriminant_zero_l()[source]¶
Method to calculate the pressure which zero the discriminant function of the general cubic eos, and is likely to sit on a boundary between not having a liquid-like volume; and having a liquid-like volume.
- Returns
- P_discriminant_zero_l
float Pressure which make the discriminants zero at the right condition, [Pa]
- P_discriminant_zero_l
Examples
>>> eos = PRTranslatedConsistent(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6) >>> P_trans = eos.P_discriminant_zero_l() >>> P_trans 478346.37289
In this case, the discriminant transition shows the change in roots:
>>> eos.to(T=eos.T, P=P_trans*.99999999).mpmath_volumes_float ((0.00013117994140177062+0j), (0.002479717165903531+0j), (0.002480236178570793+0j)) >>> eos.to(T=eos.T, P=P_trans*1.0000001).mpmath_volumes_float ((0.0001311799413872173+0j), (0.002479976386402769-8.206310112063695e-07j), (0.002479976386402769+8.206310112063695e-07j))
- P_discriminant_zeros()[source]¶
Method to calculate the pressures which zero the discriminant function of the general cubic eos, at the current temperature.
Examples
>>> eos = PRTranslatedConsistent(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6) >>> eos.P_discriminant_zeros() [478346.3, 149960391.7]
- static P_discriminant_zeros_analytical(T, b, delta, epsilon, a_alpha, valid=False)[source]¶
Method to calculate the pressures which zero the discriminant function of the general cubic eos. This is a quartic function solved analytically.
- Parameters
- T
float Temperature, [K]
- b
float Coefficient calculated by EOS-specific method, [m^3/mol]
- delta
float Coefficient calculated by EOS-specific method, [m^3/mol]
- epsilon
float Coefficient calculated by EOS-specific method, [m^6/mol^2]
- a_alpha
float Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- validbool
Whether to filter the calculated pressures so that they are all real, and positive only, [-]
- T
- Returns
- P_discriminant_zeros
float Pressures which make the discriminants zero, [Pa]
- P_discriminant_zeros
Notes
Calculated analytically. Derived as follows.
>>> from sympy import * >>> P, T, V, R, b, a, delta, epsilon = symbols('P, T, V, R, b, a, delta, epsilon') >>> eta = b >>> B = b*P/(R*T) >>> deltas = delta*P/(R*T) >>> thetas = a*P/(R*T)**2 >>> epsilons = epsilon*(P/(R*T))**2 >>> etas = eta*P/(R*T) >>> a_coeff = 1 >>> b_coeff = (deltas - B - 1) >>> c = (thetas + epsilons - deltas*(B+1)) >>> d = -(epsilons*(B+1) + thetas*etas) >>> disc = b_coeff*b_coeff*c*c - 4*a_coeff*c*c*c - 4*b_coeff*b_coeff*b_coeff*d - 27*a_coeff*a_coeff*d*d + 18*a_coeff*b_coeff*c*d >>> base = -(expand(disc/P**2*R**3*T**3)) >>> sln = collect(base, P)
- P_max_at_V(V)[source]¶
Dummy method. The idea behind this method, which is implemented by some subclasses, is to calculate the maximum pressure the EOS can create at a constant volume, if one exists; returns None otherwise. This method, as a dummy method, always returns None.
- P_zero_g_cheb_limits = (0.0, 0.0)¶
- P_zero_l_cheb_limits = (0.0, 0.0)¶
- Psat(T, polish=False, guess=None)[source]¶
Generic method to calculate vapor pressure for a specified T.
From Tc to 0.32Tc, uses a 10th order polynomial of the following form:
If polish is True, SciPy’s newton solver is launched with the calculated vapor pressure as an initial guess in an attempt to get more accuracy. This may not converge however.
Results above the critical temperature are meaningless. A first-order polynomial is used to extrapolate under 0.32 Tc; however, there is normally not a volume solution to the EOS which can produce that low of a pressure.
- Parameters
- Returns
- Psat
float Vapor pressure, [Pa]
- Psat
Notes
EOSs sharing the same b, delta, and epsilon have the same coefficient sets.
Form for the regression is inspired from [1].
No volume solution is needed when polish=False; the only external call is for the value of a_alpha.
References
- 1
Soave, G. “Direct Calculation of Pure-Compound Vapour Pressures through Cubic Equations of State.” Fluid Phase Equilibria 31, no. 2 (January 1, 1986): 203-7. doi:10.1016/0378-3812(86)90013-0.
- Psat_cheb_range = (0.0, 0.0)¶
- Psat_errors(Tmin=None, Tmax=None, pts=50, plot=False, show=False, trunc_err_low=1e-18, trunc_err_high=1.0, Pmin=1e-100)[source]¶
Method to create a plot of vapor pressure and the relative error of its calculation vs. the iterative polish approach.
- Parameters
- Tmin
float Minimum temperature of calculation; if this is too low the saturation routines will stop converging, [K]
- Tmax
float Maximum temperature of calculation; cannot be above the critical temperature, [K]
- pts
int,optional The number of temperature points to include [-]
- plotbool
If False, the solution is returned without plotting the data, [-]
- showbool
Whether or not the plot should be rendered and shown; a handle to it is returned if plot is True for other purposes such as saving the plot to a file, [-]
- trunc_err_low
float Minimum plotted error; values under this are rounded to 0, [-]
- trunc_err_high
float Maximum plotted error; values above this are rounded to 1, [-]
- Pmin
float Minimum pressure for the solution to work on, [Pa]
- Tmin
- Returns
- T_discriminant_zero_g(T_guess=None)[source]¶
Method to calculate the temperature which zeros the discriminant function of the general cubic eos, and is likely to sit on a boundary between not having a vapor-like volume; and having a vapor-like volume.
- Parameters
- T_guess
float,optional Temperature guess, [K]
- T_guess
- Returns
- T_discriminant_zero_g
float Temperature which make the discriminants zero at the right condition, [K]
- T_discriminant_zero_g
Notes
Significant numerical issues remain in improving this method.
Examples
>>> eos = PRTranslatedConsistent(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6) >>> T_trans = eos.T_discriminant_zero_g() >>> T_trans 644.3023307
In this case, the discriminant transition does not reveal a transition to two roots being available, only to there being a double (imaginary) root.
>>> eos.to(P=eos.P, T=T_trans).mpmath_volumes_float ((9.309597822372529e-05-0.00015876248805149625j), (9.309597822372529e-05+0.00015876248805149625j), (0.005064847204219234+0j))
- T_discriminant_zero_l(T_guess=None)[source]¶
Method to calculate the temperature which zeros the discriminant function of the general cubic eos, and is likely to sit on a boundary between not having a liquid-like volume; and having a liquid-like volume.
- Parameters
- T_guess
float,optional Temperature guess, [K]
- T_guess
- Returns
- T_discriminant_zero_l
float Temperature which make the discriminants zero at the right condition, [K]
- T_discriminant_zero_l
Notes
Significant numerical issues remain in improving this method.
Examples
>>> eos = PRTranslatedConsistent(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6) >>> T_trans = eos.T_discriminant_zero_l() >>> T_trans 644.3023307
In this case, the discriminant transition does not reveal a transition to two roots being available, only to there being a double (imaginary) root.
>>> eos.to(P=eos.P, T=T_trans).mpmath_volumes_float ((9.309597822372529e-05-0.00015876248805149625j), (9.309597822372529e-05+0.00015876248805149625j), (0.005064847204219234+0j))
- T_max_at_V(V, Pmax=None)[source]¶
Method to calculate the maximum temperature the EOS can create at a constant volume, if one exists; returns None otherwise.
- Parameters
- Returns
- T
float Maximum possible temperature, [K]
- T
Examples
>>> e = PR(P=1e5, V=0.0001437, Tc=512.5, Pc=8084000.0, omega=0.559) >>> e.T_max_at_V(e.V) 431155.5
- T_min_at_V(V, Pmin=1e-15)[source]¶
Returns the minimum temperature for the EOS to have the volume as specified. Under this temperature, the pressure will go negative (and the EOS will not solve).
- Tsat(P, polish=False)[source]¶
Generic method to calculate the temperature for a specified vapor pressure of the pure fluid. This is simply a bounded solver running between 0.2Tc and Tc on the Psat method.
- Parameters
- Returns
- Tsat
float Temperature of saturation, [K]
- Tsat
Notes
It is recommended not to run with polish=True, as that will make the calculation much slower.
- property U_dep_g¶
Departure molar internal energy from ideal gas behavior for the gas phase, [J/mol].
- property U_dep_l¶
Departure molar internal energy from ideal gas behavior for the liquid phase, [J/mol].
- property V_dep_g¶
Departure molar volume from ideal gas behavior for the gas phase, [m^3/mol].
- property V_dep_l¶
Departure molar volume from ideal gas behavior for the liquid phase, [m^3/mol].
- property V_g_mpmath¶
The molar volume of the gas phase calculated with mpmath to a higher precision, [m^3/mol]. This is useful for validating the cubic root solver(s). It is not quite a true arbitrary solution to the EOS, because the constants b,`epsilon`, delta and a_alpha as well as the input arguments T and P are not calculated with arbitrary precision. This is a feature when comparing the volume solution algorithms however as they work with the same finite-precision variables.
- V_g_sat(T)[source]¶
Method to calculate molar volume of the vapor phase along the saturation line.
- Parameters
- T
float Temperature, [K]
- T
- Returns
- V_g_sat
float Gas molar volume along the saturation line, [m^3/mol]
- V_g_sat
Notes
Computes Psat, and then uses volume_solutions to obtain the three possible molar volumes. The highest value is returned.
- property V_l_mpmath¶
The molar volume of the liquid phase calculated with mpmath to a higher precision, [m^3/mol]. This is useful for validating the cubic root solver(s). It is not quite a true arbitrary solution to the EOS, because the constants b,`epsilon`, delta and a_alpha as well as the input arguments T and P are not calculated with arbitrary precision. This is a feature when comparing the volume solution algorithms however as they work with the same finite-precision variables.
- V_l_sat(T)[source]¶
Method to calculate molar volume of the liquid phase along the saturation line.
- Parameters
- T
float Temperature, [K]
- T
- Returns
- V_l_sat
float Liquid molar volume along the saturation line, [m^3/mol]
- V_l_sat
Notes
Computes Psat, and then uses volume_solutions to obtain the three possible molar volumes. The lowest value is returned.
- property Vc¶
Critical volume, [m^3/mol].
- Vs_mpmath()[source]¶
Method to calculate real roots of a cubic equation, using mpmath.
- Returns
- Vs
list[mpf] Either 1 or 3 real volumes as calculated by mpmath, [m^3/mol]
- Vs
Examples
>>> eos = PRTranslatedTwu(T=300, P=1e5, Tc=512.5, Pc=8084000.0, omega=0.559, alpha_coeffs=(0.694911, 0.9199, 1.7), c=-1e-6) >>> eos.Vs_mpmath() [mpf('0.0000489261705320261435106226558966745'), mpf('0.000541508154451321441068958547812526'), mpf('0.0243149463942697410611501615357228')]
- __repr__()[source]¶
Create a string representation of the EOS - by default, include all parameters so as to make it easy to construct new instances from states. Includes the two specified state variables, Tc, Pc, omega and any kwargs.
- Returns
- recreation
str String which is valid Python and recreates the current state of the object if ran, [-]
- recreation
Examples
>>> eos = PR(Tc=507.6, Pc=3025000.0, omega=0.2975, T=400.0, P=1e6) >>> eos PR(Tc=507.6, Pc=3025000.0, omega=0.2975, T=400.0, P=1000000.0)
- a_alpha_and_derivatives(T, full=True, quick=True, pure_a_alphas=True)[source]¶
Method to calculate and its first and second derivatives.
- Parameters
- T
float Temperature, [K]
- fullbool,
optional If False, calculates and returns only a_alpha, [-]
- quickbool,
optional Legary parameter being phased out [-]
- pure_a_alphasbool,
optional Whether or not to recalculate the a_alpha terms of pure components (for the case of mixtures only) which stay the same as the composition changes (i.e in a PT flash); does nothing in the case of pure EOSs [-]
- T
- Returns
- a_alpha_and_derivatives_pure(T)[source]¶
Dummy method to calculate and its first and second derivatives. Should be implemented with the same function signature in each EOS variant; this only raises a NotImplemented Exception. Should return ‘a_alpha’, ‘da_alpha_dT’, and ‘d2a_alpha_dT2’.
- Parameters
- T
float Temperature, [K]
- T
- Returns
- a_alpha_for_Psat(T, Psat, a_alpha_guess=None)[source]¶
Method to calculate which value of is required for a given T, Psat pair. This is a numerical solution, but not a very complicated one.
- Parameters
- Returns
- a_alpha
float Value calculated to match specified volume for the current EOS, [J^2/mol^2/Pa]
- a_alpha
Notes
The implementation of this function is a direct calculation of departure gibbs energy, which is equal in both phases at saturation.
Examples
>>> eos = PR(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6) >>> eos.a_alpha_for_Psat(T=400, Psat=5e5) 3.1565798926
- a_alpha_for_V(T, P, V)[source]¶
Method to calculate which value of is required for a given T, P pair to match a specified V. This is a straightforward analytical equation.
- Parameters
- Returns
- a_alpha
float Value calculated to match specified volume for the current EOS, [J^2/mol^2/Pa]
- a_alpha
Notes
The derivation of the solution is as follows:
>>> from sympy import * >>> P, T, V, R, b, a, delta, epsilon = symbols('P, T, V, R, b, a, delta, epsilon') >>> a_alpha = symbols('a_alpha') >>> CUBIC = R*T/(V-b) - a_alpha/(V*V + delta*V + epsilon) >>> solve(Eq(CUBIC, P), a_alpha) [(-P*V**3 + P*V**2*b - P*V**2*delta + P*V*b*delta - P*V*epsilon + P*b*epsilon + R*T*V**2 + R*T*V*delta + R*T*epsilon)/(V - b)]
- a_alpha_plot(Tmin=0.0001, Tmax=None, pts=1000, plot=True, show=True)[source]¶
Method to create a plot of the parameter and its first two derivatives. This easily allows identification of EOSs which are displaying inconsistent behavior.
- Parameters
- Tmin
float Minimum temperature of calculation, [K]
- Tmax
float Maximum temperature of calculation, [K]
- pts
int,optional The number of temperature points to include [-]
- plotbool
If False, the calculated values and temperatures are returned without plotting the data, [-]
- showbool
Whether or not the plot should be rendered and shown; a handle to it is returned if plot is True for other purposes such as saving the plot to a file, [-]
- Tmin
- Returns
- Ts
list[float] Logarithmically spaced temperatures in specified range, [K]
- a_alpha
list[float] Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- da_alpha_dT
list[float] Temperature derivative of coefficient calculated by EOS-specific method, [J^2/mol^2/Pa/K]
- d2a_alpha_dT2
list[float] Second temperature derivative of coefficient calculated by EOS-specific method, [J^2/mol^2/Pa/K^2]
- fig
matplotlib.figure.Figure Plotted figure, only returned if plot is True, [-]
- Ts
- as_json()[source]¶
Method to create a JSON-friendly serialization of the eos which can be stored, and reloaded later.
- Returns
- json_repr
dict JSON-friendly representation, [-]
- json_repr
Examples
>>> import json >>> eos = MSRKTranslated(Tc=507.6, Pc=3025000, omega=0.2975, c=22.0561E-6, M=0.7446, N=0.2476, T=250., P=1E6) >>> assert eos == MSRKTranslated.from_json(json.loads(json.dumps(eos.as_json())))
- property beta_g¶
Isobaric (constant-pressure) expansion coefficient for the gas phase, [1/K].
- property beta_l¶
Isobaric (constant-pressure) expansion coefficient for the liquid phase, [1/K].
- c1 = None¶
Parameter used by some equations of state in the a calculation
- c2 = None¶
Parameter used by some equations of state in the b calculation
- check_sufficient_inputs()[source]¶
Method to an exception if none of the pairs (T, P), (T, V), or (P, V) are given.
- property d2H_dep_dT2_g¶
Second temperature derivative of departure enthalpy with respect to temperature for the gas phase, [(J/mol)/K^2].
- property d2H_dep_dT2_g_P¶
Second temperature derivative of departure enthalpy with respect to temperature for the gas phase, [(J/mol)/K^2].
- property d2H_dep_dT2_g_V¶
Second temperature derivative of departure enthalpy with respect to temperature at constant volume for the gas phase, [(J/mol)/K^2].
- property d2H_dep_dT2_l¶
Second temperature derivative of departure enthalpy with respect to temperature for the liquid phase, [(J/mol)/K^2].
- property d2H_dep_dT2_l_P¶
Second temperature derivative of departure enthalpy with respect to temperature for the liquid phase, [(J/mol)/K^2].
- property d2H_dep_dT2_l_V¶
Second temperature derivative of departure enthalpy with respect to temperature at constant volume for the liquid phase, [(J/mol)/K^2].
- property d2H_dep_dTdP_g¶
Temperature and pressure derivative of departure enthalpy at constant pressure then temperature for the gas phase, [(J/mol)/K/Pa].
- property d2H_dep_dTdP_l¶
Temperature and pressure derivative of departure enthalpy at constant pressure then temperature for the liquid phase, [(J/mol)/K/Pa].
- property d2P_dT2_PV_g¶
Second derivative of pressure with respect to temperature twice, but with pressure held constant the first time and volume held constant the second time for the gas phase, [Pa/K^2].
- property d2P_dT2_PV_l¶
Second derivative of pressure with respect to temperature twice, but with pressure held constant the first time and volume held constant the second time for the liquid phase, [Pa/K^2].
- property d2P_dTdP_g¶
Second derivative of pressure with respect to temperature and, then pressure; and with volume held constant at first, then temperature, for the gas phase, [1/K].
- property d2P_dTdP_l¶
Second derivative of pressure with respect to temperature and, then pressure; and with volume held constant at first, then temperature, for the liquid phase, [1/K].
- property d2P_dTdrho_g¶
Derivative of pressure with respect to molar density, and temperature for the gas phase, [Pa/(K*mol/m^3)].
- property d2P_dTdrho_l¶
Derivative of pressure with respect to molar density, and temperature for the liquid phase, [Pa/(K*mol/m^3)].
- property d2P_dVdP_g¶
Second derivative of pressure with respect to molar volume and then pressure for the gas phase, [mol/m^3].
- property d2P_dVdP_l¶
Second derivative of pressure with respect to molar volume and then pressure for the liquid phase, [mol/m^3].
- property d2P_dVdT_TP_g¶
Second derivative of pressure with respect to molar volume and then temperature at constant temperature then pressure for the gas phase, [Pa*mol/m^3/K].
- property d2P_dVdT_TP_l¶
Second derivative of pressure with respect to molar volume and then temperature at constant temperature then pressure for the liquid phase, [Pa*mol/m^3/K].
- property d2P_dVdT_g¶
Alias of
GCEOS.d2P_dTdV_g
- property d2P_dVdT_l¶
Alias of
GCEOS.d2P_dTdV_l
- property d2P_drho2_g¶
Second derivative of pressure with respect to molar density for the gas phase, [Pa/(mol/m^3)^2].
- property d2P_drho2_l¶
Second derivative of pressure with respect to molar density for the liquid phase, [Pa/(mol/m^3)^2].
- property d2S_dep_dT2_g¶
Second temperature derivative of departure entropy with respect to temperature for the gas phase, [(J/mol)/K^3].
- property d2S_dep_dT2_g_V¶
Second temperature derivative of departure entropy with respect to temperature at constant volume for the gas phase, [(J/mol)/K^3].
- property d2S_dep_dT2_l¶
Second temperature derivative of departure entropy with respect to temperature for the liquid phase, [(J/mol)/K^3].
- property d2S_dep_dT2_l_V¶
Second temperature derivative of departure entropy with respect to temperature at constant volume for the liquid phase, [(J/mol)/K^3].
- property d2S_dep_dTdP_g¶
Temperature and pressure derivative of departure entropy at constant pressure then temperature for the gas phase, [(J/mol)/K^2/Pa].
- property d2S_dep_dTdP_l¶
Temperature and pressure derivative of departure entropy at constant pressure then temperature for the liquid phase, [(J/mol)/K^2/Pa].
- property d2T_dP2_g¶
Second partial derivative of temperature with respect to pressure (constant volume) for the gas phase, [K/Pa^2].
- property d2T_dP2_l¶
Second partial derivative of temperature with respect to pressure (constant temperature) for the liquid phase, [K/Pa^2].
- property d2T_dPdV_g¶
Second partial derivative of temperature with respect to pressure (constant volume) and then volume (constant pressure) for the gas phase, [K*mol/(Pa*m^3)].
- property d2T_dPdV_l¶
Second partial derivative of temperature with respect to pressure (constant volume) and then volume (constant pressure) for the liquid phase, [K*mol/(Pa*m^3)].
- property d2T_dPdrho_g¶
Derivative of temperature with respect to molar density, and pressure for the gas phase, [K/(Pa*mol/m^3)].
- property d2T_dPdrho_l¶
Derivative of temperature with respect to molar density, and pressure for the liquid phase, [K/(Pa*mol/m^3)].
- property d2T_dV2_g¶
Second partial derivative of temperature with respect to volume (constant pressure) for the gas phase, [K*mol^2/m^6].
- property d2T_dV2_l¶
Second partial derivative of temperature with respect to volume (constant pressure) for the liquid phase, [K*mol^2/m^6].
- property d2T_dVdP_g¶
Second partial derivative of temperature with respect to pressure (constant volume) and then volume (constant pressure) for the gas phase, [K*mol/(Pa*m^3)].
- property d2T_dVdP_l¶
Second partial derivative of temperature with respect to pressure (constant volume) and then volume (constant pressure) for the liquid phase, [K*mol/(Pa*m^3)].
- property d2T_drho2_g¶
Second derivative of temperature with respect to molar density for the gas phase, [K/(mol/m^3)^2].
- property d2T_drho2_l¶
Second derivative of temperature with respect to molar density for the liquid phase, [K/(mol/m^3)^2].
- property d2V_dP2_g¶
Second partial derivative of volume with respect to pressure (constant temperature) for the gas phase, [m^3/(Pa^2*mol)].
- property d2V_dP2_l¶
Second partial derivative of volume with respect to pressure (constant temperature) for the liquid phase, [m^3/(Pa^2*mol)].
- property d2V_dPdT_g¶
Second partial derivative of volume with respect to pressure (constant temperature) and then presssure (constant temperature) for the gas phase, [m^3/(K*Pa*mol)].
- property d2V_dPdT_l¶
Second partial derivative of volume with respect to pressure (constant temperature) and then presssure (constant temperature) for the liquid phase, [m^3/(K*Pa*mol)].
- property d2V_dT2_g¶
Second partial derivative of volume with respect to temperature (constant pressure) for the gas phase, [m^3/(mol*K^2)].
- property d2V_dT2_l¶
Second partial derivative of volume with respect to temperature (constant pressure) for the liquid phase, [m^3/(mol*K^2)].
- property d2V_dTdP_g¶
Second partial derivative of volume with respect to pressure (constant temperature) and then presssure (constant temperature) for the gas phase, [m^3/(K*Pa*mol)].
- property d2V_dTdP_l¶
Second partial derivative of volume with respect to pressure (constant temperature) and then presssure (constant temperature) for the liquid phase, [m^3/(K*Pa*mol)].
- property d2a_alpha_dTdP_g_V¶
Derivative of the temperature derivative of a_alpha with respect to pressure at constant volume (varying T) for the gas phase, [J^2/mol^2/Pa^2/K].
- property d2a_alpha_dTdP_l_V¶
Derivative of the temperature derivative of a_alpha with respect to pressure at constant volume (varying T) for the liquid phase, [J^2/mol^2/Pa^2/K].
- d2phi_sat_dT2(T, polish=True)[source]¶
Method to calculate the second temperature derivative of saturation fugacity coefficient of the compound. This does require solving the EOS itself.
- Parameters
- Returns
- d2phi_sat_dT2
float Second temperature derivative of fugacity coefficient along the liquid-vapor saturation line, [1/K^2]
- d2phi_sat_dT2
Notes
This is presently a numerical calculation.
- property d2rho_dP2_g¶
Second derivative of molar density with respect to pressure for the gas phase, [(mol/m^3)/Pa^2].
- property d2rho_dP2_l¶
Second derivative of molar density with respect to pressure for the liquid phase, [(mol/m^3)/Pa^2].
- property d2rho_dPdT_g¶
Second derivative of molar density with respect to pressure and temperature for the gas phase, [(mol/m^3)/(K*Pa)].
- property d2rho_dPdT_l¶
Second derivative of molar density with respect to pressure and temperature for the liquid phase, [(mol/m^3)/(K*Pa)].
- property d2rho_dT2_g¶
Second derivative of molar density with respect to temperature for the gas phase, [(mol/m^3)/K^2].
- property d2rho_dT2_l¶
Second derivative of molar density with respect to temperature for the liquid phase, [(mol/m^3)/K^2].
- property d3a_alpha_dT3¶
Method to calculate the third temperature derivative of , [J^2/mol^2/Pa/K^3]. This parameter is needed for some higher derivatives that are needed in some flash calculations.
- Returns
- d3a_alpha_dT3
float Third temperature derivative of coefficient calculated by EOS-specific method, [J^2/mol^2/Pa/K^3]
- d3a_alpha_dT3
- property dH_dep_dP_g¶
Derivative of departure enthalpy with respect to pressure for the gas phase, [(J/mol)/Pa].
- property dH_dep_dP_g_V¶
Derivative of departure enthalpy with respect to pressure at constant volume for the liquid phase, [(J/mol)/Pa].
- property dH_dep_dP_l¶
Derivative of departure enthalpy with respect to pressure for the liquid phase, [(J/mol)/Pa].
- property dH_dep_dP_l_V¶
Derivative of departure enthalpy with respect to pressure at constant volume for the gas phase, [(J/mol)/Pa].
- property dH_dep_dT_g¶
Derivative of departure enthalpy with respect to temperature for the gas phase, [(J/mol)/K].
- property dH_dep_dT_g_V¶
Derivative of departure enthalpy with respect to temperature at constant volume for the gas phase, [(J/mol)/K].
- property dH_dep_dT_l¶
Derivative of departure enthalpy with respect to temperature for the liquid phase, [(J/mol)/K].
- property dH_dep_dT_l_V¶
Derivative of departure enthalpy with respect to temperature at constant volume for the liquid phase, [(J/mol)/K].
- dH_dep_dT_sat_g(T, polish=False)[source]¶
Method to calculate and return the temperature derivative of saturation vapor excess enthalpy.
- dH_dep_dT_sat_l(T, polish=False)[source]¶
Method to calculate and return the temperature derivative of saturation liquid excess enthalpy.
- property dH_dep_dV_g_P¶
Derivative of departure enthalpy with respect to volume at constant pressure for the gas phase, [J/m^3].
- property dH_dep_dV_g_T¶
Derivative of departure enthalpy with respect to volume at constant temperature for the gas phase, [J/m^3].
- property dH_dep_dV_l_P¶
Derivative of departure enthalpy with respect to volume at constant pressure for the liquid phase, [J/m^3].
- property dH_dep_dV_l_T¶
Derivative of departure enthalpy with respect to volume at constant temperature for the gas phase, [J/m^3].
- property dP_drho_g¶
Derivative of pressure with respect to molar density for the gas phase, [Pa/(mol/m^3)].
- property dP_drho_l¶
Derivative of pressure with respect to molar density for the liquid phase, [Pa/(mol/m^3)].
- dPsat_dT(T, polish=False, also_Psat=False)[source]¶
Generic method to calculate the temperature derivative of vapor pressure for a specified T. Implements the analytical derivative of the three polynomials described in Psat.
As with Psat, results above the critical temperature are meaningless. The first-order polynomial which is used to calculate it under 0.32 Tc may not be physicall meaningful, due to there normally not being a volume solution to the EOS which can produce that low of a pressure.
- Parameters
- T
float Temperature, [K]
- polishbool,
optional Whether to attempt to use a numerical solver to make the solution more precise or not
- also_Psatbool,
optional Calculating dPsat_dT necessarily involves calculating Psat; when this is set to True, a second return value is added, whic is the actual Psat value.
- T
- Returns
Notes
There is a small step change at 0.32 Tc for all EOS due to the two switch between polynomials at that point.
Useful for calculating enthalpy of vaporization with the Clausius Clapeyron Equation. Derived with SymPy’s diff and cse.
- property dS_dep_dP_g¶
Derivative of departure entropy with respect to pressure for the gas phase, [(J/mol)/K/Pa].
- property dS_dep_dP_g_V¶
Derivative of departure entropy with respect to pressure at constant volume for the gas phase, [(J/mol)/K/Pa].
- property dS_dep_dP_l¶
Derivative of departure entropy with respect to pressure for the liquid phase, [(J/mol)/K/Pa].
- property dS_dep_dP_l_V¶
Derivative of departure entropy with respect to pressure at constant volume for the liquid phase, [(J/mol)/K/Pa].
- property dS_dep_dT_g¶
Derivative of departure entropy with respect to temperature for the gas phase, [(J/mol)/K^2].
- property dS_dep_dT_g_V¶
Derivative of departure entropy with respect to temperature at constant volume for the gas phase, [(J/mol)/K^2].
- property dS_dep_dT_l¶
Derivative of departure entropy with respect to temperature for the liquid phase, [(J/mol)/K^2].
- property dS_dep_dT_l_V¶
Derivative of departure entropy with respect to temperature at constant volume for the liquid phase, [(J/mol)/K^2].
- dS_dep_dT_sat_g(T, polish=False)[source]¶
Method to calculate and return the temperature derivative of saturation vapor excess entropy.
- dS_dep_dT_sat_l(T, polish=False)[source]¶
Method to calculate and return the temperature derivative of saturation liquid excess entropy.
- property dS_dep_dV_g_P¶
Derivative of departure entropy with respect to volume at constant pressure for the gas phase, [J/K/m^3].
- property dS_dep_dV_g_T¶
Derivative of departure entropy with respect to volume at constant temperature for the gas phase, [J/K/m^3].
- property dS_dep_dV_l_P¶
Derivative of departure entropy with respect to volume at constant pressure for the liquid phase, [J/K/m^3].
- property dS_dep_dV_l_T¶
Derivative of departure entropy with respect to volume at constant temperature for the gas phase, [J/K/m^3].
- property dT_drho_g¶
Derivative of temperature with respect to molar density for the gas phase, [K/(mol/m^3)].
- property dT_drho_l¶
Derivative of temperature with respect to molar density for the liquid phase, [K/(mol/m^3)].
- property dZ_dP_g¶
Derivative of compressibility factor with respect to pressure for the gas phase, [1/Pa].
- property dZ_dP_l¶
Derivative of compressibility factor with respect to pressure for the liquid phase, [1/Pa].
- property dZ_dT_g¶
Derivative of compressibility factor with respect to temperature for the gas phase, [1/K].
- property dZ_dT_l¶
Derivative of compressibility factor with respect to temperature for the liquid phase, [1/K].
- property da_alpha_dP_g_V¶
Derivative of the a_alpha with respect to pressure at constant volume (varying T) for the gas phase, [J^2/mol^2/Pa^2].
- property da_alpha_dP_l_V¶
Derivative of the a_alpha with respect to pressure at constant volume (varying T) for the liquid phase, [J^2/mol^2/Pa^2].
- property dbeta_dP_g¶
Derivative of isobaric expansion coefficient with respect to pressure for the gas phase, [1/(Pa*K)].
- property dbeta_dP_l¶
Derivative of isobaric expansion coefficient with respect to pressure for the liquid phase, [1/(Pa*K)].
- property dbeta_dT_g¶
Derivative of isobaric expansion coefficient with respect to temperature for the gas phase, [1/K^2].
- property dbeta_dT_l¶
Derivative of isobaric expansion coefficient with respect to temperature for the liquid phase, [1/K^2].
- property dfugacity_dP_g¶
Derivative of fugacity with respect to pressure for the gas phase, [-].
- property dfugacity_dP_l¶
Derivative of fugacity with respect to pressure for the liquid phase, [-].
- property dfugacity_dT_g¶
Derivative of fugacity with respect to temperature for the gas phase, [Pa/K].
- property dfugacity_dT_l¶
Derivative of fugacity with respect to temperature for the liquid phase, [Pa/K].
- discriminant(T=None, P=None)[source]¶
Method to compute the discriminant of the cubic volume solution with the current EOS parameters, optionally at the same (assumed) T, and P or at different ones, if values are specified.
- Parameters
- Returns
- discriminant
float Discriminant, [-]
- discriminant
Notes
This call is quite quick; only is needed and if T is the same as the current object than it has already been computed.
The formula is as follows:
The formula is derived as follows:
>>> from sympy import * >>> P, T, R, b = symbols('P, T, R, b') >>> a_alpha = symbols(r'a\ \alpha', cls=Function) >>> delta, epsilon = symbols('delta, epsilon') >>> eta = b >>> B = b*P/(R*T) >>> deltas = delta*P/(R*T) >>> thetas = a_alpha(T)*P/(R*T)**2 >>> epsilons = epsilon*(P/(R*T))**2 >>> etas = eta*P/(R*T) >>> a = 1 >>> b = (deltas - B - 1) >>> c = (thetas + epsilons - deltas*(B+1)) >>> d = -(epsilons*(B+1) + thetas*etas) >>> disc = b*b*c*c - 4*a*c*c*c - 4*b*b*b*d - 27*a*a*d*d + 18*a*b*c*d
Examples
>>> base = PR(Tc=507.6, Pc=3025000.0, omega=0.2975, T=500.0, P=1E6) >>> base.discriminant() -0.001026390999 >>> base.discriminant(T=400) 0.0010458828 >>> base.discriminant(T=400, P=1e9) 12584660355.4
- property dphi_dP_g¶
Derivative of fugacity coefficient with respect to pressure for the gas phase, [1/Pa].
- property dphi_dP_l¶
Derivative of fugacity coefficient with respect to pressure for the liquid phase, [1/Pa].
- property dphi_dT_g¶
Derivative of fugacity coefficient with respect to temperature for the gas phase, [1/K].
- property dphi_dT_l¶
Derivative of fugacity coefficient with respect to temperature for the liquid phase, [1/K].
- dphi_sat_dT(T, polish=True)[source]¶
Method to calculate the temperature derivative of saturation fugacity coefficient of the compound. This does require solving the EOS itself.
- property drho_dP_g¶
Derivative of molar density with respect to pressure for the gas phase, [(mol/m^3)/Pa].
- property drho_dP_l¶
Derivative of molar density with respect to pressure for the liquid phase, [(mol/m^3)/Pa].
- property drho_dT_g¶
Derivative of molar density with respect to temperature for the gas phase, [(mol/m^3)/K].
- property drho_dT_l¶
Derivative of molar density with respect to temperature for the liquid phase, [(mol/m^3)/K].
- classmethod from_json(json_repr)[source]¶
Method to create a eos from a JSON serialization of another eos.
- Parameters
- json_repr
dict JSON-friendly representation, [-]
- json_repr
- Returns
- eos
GCEOS Newly created object from the json serialization, [-]
- eos
Notes
It is important that the input string be in the same format as that created by
GCEOS.as_json.Examples
>>> eos = MSRKTranslated(Tc=507.6, Pc=3025000, omega=0.2975, c=22.0561E-6, M=0.7446, N=0.2476, T=250., P=1E6) >>> string = eos.as_json() >>> new_eos = GCEOS.from_json(string) >>> assert eos.__dict__ == new_eos.__dict__
- property fugacity_g¶
Fugacity for the gas phase, [Pa].
- property fugacity_l¶
Fugacity for the liquid phase, [Pa].
- property kappa_g¶
Isothermal (constant-temperature) expansion coefficient for the gas phase, [1/Pa].
- property kappa_l¶
Isothermal (constant-temperature) expansion coefficient for the liquid phase, [1/Pa].
- kwargs = {}¶
Dictionary which holds input parameters to an EOS which are non-standard; this excludes T, P, V, omega, Tc, Pc, Vc but includes EOS specific parameters like S1 and alpha_coeffs.
- kwargs_keys = ()¶
- property lnphi_g¶
The natural logarithm of the fugacity coefficient for the gas phase, [-].
- property lnphi_l¶
The natural logarithm of the fugacity coefficient for the liquid phase, [-].
- model_hash()[source]¶
Basic method to calculate a hash of the non-state parts of the model This is useful for comparing to models to determine if they are the same, i.e. in a VLL flash it is important to know if both liquids have the same model.
Note that the hashes should only be compared on the same system running in the same process!
- Returns
- model_hash
int Hash of the object’s model parameters, [-]
- model_hash
- property more_stable_phase¶
Checks the Gibbs energy of each possible phase, and returns ‘l’ if the liquid-like phase is more stable, and ‘g’ if the vapor-like phase is more stable.
Examples
>>> PR(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6).more_stable_phase 'l'
- property mpmath_volume_ratios¶
Method to compare, as ratios, the volumes of the implemented cubic solver versus those calculated using mpmath.
- Returns
- ratios
list[mpc] Either 1 or 3 volume ratios as calculated by mpmath, [-]
- ratios
Examples
>>> eos = PRTranslatedTwu(T=300, P=1e5, Tc=512.5, Pc=8084000.0, omega=0.559, alpha_coeffs=(0.694911, 0.9199, 1.7), c=-1e-6) >>> eos.mpmath_volume_ratios (mpc(real='0.99999999999999995', imag='0.0'), mpc(real='0.999999999999999965', imag='0.0'), mpc(real='1.00000000000000005', imag='0.0'))
- property mpmath_volumes¶
Method to calculate to a high precision the exact roots to the cubic equation, using mpmath.
- Returns
- Vs
tuple[mpf] 3 Real or not real volumes as calculated by mpmath, [m^3/mol]
- Vs
Examples
>>> eos = PRTranslatedTwu(T=300, P=1e5, Tc=512.5, Pc=8084000.0, omega=0.559, alpha_coeffs=(0.694911, 0.9199, 1.7), c=-1e-6) >>> eos.mpmath_volumes (mpf('0.0000489261705320261435106226558966745'), mpf('0.000541508154451321441068958547812526'), mpf('0.0243149463942697410611501615357228'))
- property mpmath_volumes_float¶
Method to calculate real roots of a cubic equation, using mpmath, but returned as floats.
Examples
>>> eos = PRTranslatedTwu(T=300, P=1e5, Tc=512.5, Pc=8084000.0, omega=0.559, alpha_coeffs=(0.694911, 0.9199, 1.7), c=-1e-6) >>> eos.mpmath_volumes_float ((4.892617053202614e-05+0j), (0.0005415081544513214+0j), (0.024314946394269742+0j))
- multicomponent = False¶
Whether or not the EOS is multicomponent or not
- nonstate_constants = ('Tc', 'Pc', 'omega', 'kwargs', 'a', 'b', 'delta', 'epsilon')¶
- property phi_g¶
Fugacity coefficient for the gas phase, [Pa].
- property phi_l¶
Fugacity coefficient for the liquid phase, [Pa].
- phi_sat(T, polish=True)[source]¶
Method to calculate the saturation fugacity coefficient of the compound. This does not require solving the EOS itself.
- Parameters
- Returns
- phi_sat
float Fugacity coefficient along the liquid-vapor saturation line, [-]
- phi_sat
Notes
Accuracy is generally around 1e-7. If Tr is under 0.32, the rigorous method is always used, but a solution may not exist if both phases cannot coexist. If Tr is above 1, likewise a solution does not exist.
- resolve_full_alphas()[source]¶
Generic method to resolve the eos with fully calculated alpha derviatives. Re-calculates properties with the new alpha derivatives for any previously solved roots.
- property rho_g¶
Gas molar density, [mol/m^3].
- property rho_l¶
Liquid molar density, [mol/m^3].
- saturation_prop_plot(prop, Tmin=None, Tmax=None, pts=100, plot=False, show=False, both=False)[source]¶
Method to create a plot of a specified property of the EOS along the (pure component) saturation line.
- Parameters
- prop
str Property to be used; such as ‘H_dep_l’ ( when both is False) or ‘H_dep’ (when both is True), [-]
- Tmin
float Minimum temperature of calculation; if this is too low the saturation routines will stop converging, [K]
- Tmax
float Maximum temperature of calculation; cannot be above the critical temperature, [K]
- pts
int,optional The number of temperature points to include [-]
- plotbool
If False, the calculated values and temperatures are returned without plotting the data, [-]
- showbool
Whether or not the plot should be rendered and shown; a handle to it is returned if plot is True for other purposes such as saving the plot to a file, [-]
- bothbool
When true, append ‘_l’ and ‘_g’ and draw both the liquid and vapor property specified and return two different sets of values.
- prop
- Returns
- Ts
list[float] Logarithmically spaced temperatures in specified range, [K]
- props
list[float] The property specified if both is False; otherwise, the liquid properties, [various]
- props_g
list[float] The gas properties, only returned if both is True, [various]
- fig
matplotlib.figure.Figure Plotted figure, only returned if plot is True, [-]
- Ts
- scalar = True¶
- set_from_PT(Vs, only_l=False, only_g=False)[source]¶
Counts the number of real volumes in Vs, and determines what to do. If there is only one real volume, the method set_properties_from_solution is called with it. If there are two real volumes, set_properties_from_solution is called once with each volume. The phase is returned by set_properties_from_solution, and the volumes is set to either V_l or V_g as appropriate.
- Parameters
Notes
An optimization attempt was made to remove min() and max() from this function; that is indeed possible, but the check for handling if there are two or three roots makes it not worth it.
- set_properties_from_solution(T, P, V, b, delta, epsilon, a_alpha, da_alpha_dT, d2a_alpha_dT2, force_l=False, force_g=False)[source]¶
Sets all interesting properties which can be calculated from an EOS alone. Determines which phase the fluid is on its own; for details, see phase_identification_parameter.
The list of properties set is as follows, with all properties suffixed with ‘_l’ or ‘_g’.
dP_dT, dP_dV, dV_dT, dV_dP, dT_dV, dT_dP, d2P_dT2, d2P_dV2, d2V_dT2, d2V_dP2, d2T_dV2, d2T_dP2, d2V_dPdT, d2P_dTdV, d2T_dPdV, H_dep, S_dep, G_dep and PIP.
- Parameters
- T
float Temperature, [K]
- P
float Pressure, [Pa]
- V
float Molar volume, [m^3/mol]
- b
float Coefficient calculated by EOS-specific method, [m^3/mol]
- delta
float Coefficient calculated by EOS-specific method, [m^3/mol]
- epsilon
float Coefficient calculated by EOS-specific method, [m^6/mol^2]
- a_alpha
float Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- da_alpha_dT
float Temperature derivative of coefficient calculated by EOS-specific method, [J^2/mol^2/Pa/K]
- d2a_alpha_dT2
float Second temperature derivative of coefficient calculated by EOS-specific method, [J^2/mol^2/Pa/K**2]
- T
- Returns
- phase
str Either ‘l’ or ‘g’
- phase
Notes
The individual formulas for the derivatives and excess properties are as follows. For definitions of beta, see isobaric_expansion; for kappa, see isothermal_compressibility; for Cp_minus_Cv, see Cp_minus_Cv; for phase_identification_parameter, see phase_identification_parameter.
First derivatives; in part using the Triple Product Rule [2], [3]:
Second derivatives with respect to one variable; those of T and V use identities shown in [1] and verified numerically:
Second derivatives with respect to the other two variables; those of T and V use identities shown in [1] and verified numerically:
Excess properties
References
- 1(1,2)
Thorade, Matthis, and Ali Saadat. “Partial Derivatives of Thermodynamic State Properties for Dynamic Simulation.” Environmental Earth Sciences 70, no. 8 (April 10, 2013): 3497-3503. doi:10.1007/s12665-013-2394-z.
- 2
Poling, Bruce E. The Properties of Gases and Liquids. 5th edition. New York: McGraw-Hill Professional, 2000.
- 3
Walas, Stanley M. Phase Equilibria in Chemical Engineering. Butterworth-Heinemann, 1985.
- solve(pure_a_alphas=True, only_l=False, only_g=False, full_alphas=True)[source]¶
First EOS-generic method; should be called by all specific EOSs. For solving for T, the EOS must provide the method solve_T. For all cases, the EOS must provide a_alpha_and_derivatives. Calls set_from_PT once done.
- solve_T(P, V, solution=None)[source]¶
Generic method to calculate T from a specified P and V. Provides SciPy’s newton solver, and iterates to solve the general equation for P, recalculating a_alpha as a function of temperature using a_alpha_and_derivatives each iteration.
- Parameters
- Returns
- T
float Temperature, [K]
- T
- solve_missing_volumes()[source]¶
Generic method to ensure both volumes, if solutions are physical, have calculated properties. This effectively un-does the optimization of the only_l and only_g keywords.
- property sorted_volumes¶
List of lexicographically-sorted molar volumes available from the root finding algorithm used to solve the PT point. The convention of sorting lexicographically comes from numpy’s handling of complex numbers, which python does not define. This method was added to facilitate testing, as the volume solution method changes over time and the ordering does as well.
Examples
>>> PR(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6).sorted_volumes ((0.000130222125139+0j), (0.00112363131346-0.00129269672343j), (0.00112363131346+0.00129269672343j))
- state_hash()[source]¶
Basic method to calculate a hash of the state of the model and its model parameters.
Note that the hashes should only be compared on the same system running in the same process!
- Returns
- state_hash
int Hash of the object’s model parameters and state, [-]
- state_hash
- property state_specs¶
Convenience method to return the two specified state specs (T, P, or V) as a dictionary.
Examples
>>> PR(Tc=507.6, Pc=3025000.0, omega=0.2975, T=500.0, V=1.0).state_specs {'T': 500.0, 'V': 1.0}
- to(T=None, P=None, V=None)[source]¶
Method to construct a new EOS object at two of T, P or V. In the event the specs match those of the current object, it will be returned unchanged.
- Parameters
- Returns
- obj
EOS Pure component EOS at the two specified specs, [-]
- obj
Notes
Constructs the object with parameters Tc, Pc, omega, and kwargs.
Examples
>>> base = PR(Tc=507.6, Pc=3025000.0, omega=0.2975, T=500.0, P=1E6) >>> base.to(T=300.0, P=1e9).state_specs {'T': 300.0, 'P': 1000000000.0} >>> base.to(T=300.0, V=1.0).state_specs {'T': 300.0, 'V': 1.0} >>> base.to(P=1e5, V=1.0).state_specs {'P': 100000.0, 'V': 1.0}
- to_PV(P, V)[source]¶
Method to construct a new EOS object at the spcified P and V. In the event the P and V match the current object’s P and V, it will be returned unchanged.
- Parameters
- Returns
- obj
EOS Pure component EOS at specified P and V, [-]
- obj
Notes
Constructs the object with parameters Tc, Pc, omega, and kwargs.
Examples
>>> base = PR(Tc=507.6, Pc=3025000.0, omega=0.2975, T=500.0, P=1E6) >>> new = base.to_PV(P=1000.0, V=1.0) >>> base.state_specs, new.state_specs ({'T': 500.0, 'P': 1000000.0}, {'P': 1000.0, 'V': 1.0})
- to_TP(T, P)[source]¶
Method to construct a new EOS object at the spcified T and P. In the event the T and P match the current object’s T and P, it will be returned unchanged.
- Parameters
- Returns
- obj
EOS Pure component EOS at specified T and P, [-]
- obj
Notes
Constructs the object with parameters Tc, Pc, omega, and kwargs.
Examples
>>> base = PR(Tc=507.6, Pc=3025000.0, omega=0.2975, T=500.0, P=1E6) >>> new = base.to_TP(T=1.0, P=2.0) >>> base.state_specs, new.state_specs ({'T': 500.0, 'P': 1000000.0}, {'T': 1.0, 'P': 2.0})
- to_TV(T, V)[source]¶
Method to construct a new EOS object at the spcified T and V. In the event the T and V match the current object’s T and V, it will be returned unchanged.
- Parameters
- Returns
- obj
EOS Pure component EOS at specified T and V, [-]
- obj
Notes
Constructs the object with parameters Tc, Pc, omega, and kwargs.
Examples
>>> base = PR(Tc=507.6, Pc=3025000.0, omega=0.2975, T=500.0, P=1E6) >>> new = base.to_TV(T=1000000.0, V=1.0) >>> base.state_specs, new.state_specs ({'T': 500.0, 'P': 1000000.0}, {'T': 1000000.0, 'V': 1.0})
- volume_error()[source]¶
Method to calculate the relative absolute error in the calculated molar volumes. This is computed with mpmath. If the number of real roots is different between mpmath and the implemented solver, an error of 1 is returned.
Examples
>>> eos = PRTranslatedTwu(T=300, P=1e5, Tc=512.5, Pc=8084000.0, omega=0.559, alpha_coeffs=(0.694911, 0.9199, 1.7), c=-1e-6) >>> eos.volume_error() 5.2192e-17
- volume_errors(Tmin=0.0001, Tmax=10000.0, Pmin=0.01, Pmax=1000000000.0, pts=50, plot=False, show=False, trunc_err_low=1e-18, trunc_err_high=1.0, color_map=None, timing=False)[source]¶
Method to create a plot of the relative absolute error in the cubic volume solution as compared to a higher-precision calculation. This method is incredible valuable for the development of more reliable floating-point based cubic solutions.
- Parameters
- Tmin
float Minimum temperature of calculation, [K]
- Tmax
float Maximum temperature of calculation, [K]
- Pmin
float Minimum pressure of calculation, [Pa]
- Pmax
float Maximum pressure of calculation, [Pa]
- pts
int,optional The number of points to include in both the x and y axis; the validation calculation is slow, so increasing this too much is not advisable, [-]
- plotbool
If False, the calculated errors are returned without plotting the data, [-]
- showbool
Whether or not the plot should be rendered and shown; a handle to it is returned if plot is True for other purposes such as saving the plot to a file, [-]
- trunc_err_low
float Minimum plotted error; values under this are rounded to 0, [-]
- trunc_err_high
float Maximum plotted error; values above this are rounded to 1, [-]
- color_map
matplotlib.cm.ListedColormap Matplotlib colormap object, [-]
- timingbool
If True, plots the time taken by the volume root calculations themselves; this can reveal whether the solvers are taking fast or slow paths quickly, [-]
- Tmin
- Returns
- errors
list[list[float]] Relative absolute errors in the volume calculation (or timings in seconds if timing is True), [-]
- fig
matplotlib.figure.Figure Plotted figure, only returned if plot is True, [-]
- errors
- static volume_solutions(T, P, b, delta, epsilon, a_alpha)¶
Halley’s method based solver for cubic EOS volumes based on the idea of initializing from a single liquid-like guess which is solved precisely, deflating the cubic analytically, solving the quadratic equation for the next two volumes, and then performing two halley steps on each of them to obtain the final solutions. This method does not calculate imaginary roots - they are set to zero on detection. This method has been rigorously tested over a wide range of conditions.
The method uses the standard combination of bisection to provide high and low boundaries as well, to keep the iteration always moving forward.
- Parameters
- T
float Temperature, [K]
- P
float Pressure, [Pa]
- b
float Coefficient calculated by EOS-specific method, [m^3/mol]
- delta
float Coefficient calculated by EOS-specific method, [m^3/mol]
- epsilon
float Coefficient calculated by EOS-specific method, [m^6/mol^2]
- a_alpha
float Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- T
- Returns
Notes
A sample region where this method works perfectly is shown below:
- static volume_solutions_full(T, P, b, delta, epsilon, a_alpha, tries=0)¶
Newton-Raphson based solver for cubic EOS volumes based on the idea of initializing from an analytical solver. This algorithm can only be described as a monstrous mess. It is fairly fast for most cases, but about 3x slower than
volume_solutions_halley. In the worst case this will fall back to mpmath.- Parameters
- T
float Temperature, [K]
- P
float Pressure, [Pa]
- b
float Coefficient calculated by EOS-specific method, [m^3/mol]
- delta
float Coefficient calculated by EOS-specific method, [m^3/mol]
- epsilon
float Coefficient calculated by EOS-specific method, [m^6/mol^2]
- a_alpha
float Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- tries
int,optional Internal parameter as this function will call itself if it needs to; number of previous solve attempts, [-]
- T
- Returns
Notes
Sample regions where this method works perfectly are shown below:
- static volume_solutions_mp(T, P, b, delta, epsilon, a_alpha, dps=50)¶
Solution of this form of the cubic EOS in terms of volumes, using the mpmath arbitrary precision library. The number of decimal places returned is controlled by the dps parameter.
This function is the reference implementation which provides exactly correct solutions; other algorithms are compared against this one.
- Parameters
- T
float Temperature, [K]
- P
float Pressure, [Pa]
- b
float Coefficient calculated by EOS-specific method, [m^3/mol]
- delta
float Coefficient calculated by EOS-specific method, [m^3/mol]
- epsilon
float Coefficient calculated by EOS-specific method, [m^6/mol^2]
- a_alpha
float Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- dps
int Number of decimal places in the result by mpmath, [-]
- T
- Returns
Notes
Although mpmath has a cubic solver, it has been found to fail to solve in some cases. Accordingly, the algorithm is as follows:
Working precision is dps plus 40 digits; and if P < 1e-10 Pa, it is dps plus 400 digits. The input parameters are converted exactly to mpf objects on input.
polyroots from mpmath is used with maxsteps=2000, and extra precision of 15 digits. If the solution does not converge, 20 extra digits are added up to 8 times. If no solution is found, mpmath’s findroot is called on the pressure error function using three initial guesses from another solver.
Needless to say, this function is quite slow.
References
- 1
Johansson, Fredrik. Mpmath: A Python Library for Arbitrary-Precision Floating-Point Arithmetic, 2010.
Examples
Test case which presented issues for PR EOS (three roots were not being returned):
>>> volume_solutions_mpmath(0.01, 1e-05, 2.5405184201558786e-05, 5.081036840311757e-05, -6.454233843151321e-10, 0.3872747173781095) (mpf('0.0000254054613415548712260258773060137'), mpf('4.66038025602155259976574392093252'), mpf('8309.80218708657190094424659859346'))
Standard Peng-Robinson Family EOSs¶
Standard Peng Robinson¶
- class thermo.eos.PR(Tc, Pc, omega, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos.GCEOSClass for solving the Peng-Robinson [1] [2] cubic equation of state for a pure compound. Subclasses
GCEOS, which provides the methods for solving the EOS and calculating its assorted relevant thermodynamic properties. Solves the EOS on initialization.The main methods here are
PR.a_alpha_and_derivatives_pure, which calculates and its first and second derivatives, andPR.solve_T, which from a specified P and V obtains T.Two of (T, P, V) are needed to solve the EOS.
- Parameters
Notes
The constants in the expresions for a and b are given to full precision in the actual code, as derived in [3].
The full expression for critical compressibility is:
References
- 1
Peng, Ding-Yu, and Donald B. Robinson. “A New Two-Constant Equation of State.” Industrial & Engineering Chemistry Fundamentals 15, no. 1 (February 1, 1976): 59-64. doi:10.1021/i160057a011.
- 2
Robinson, Donald B., Ding-Yu Peng, and Samuel Y-K Chung. “The Development of the Peng - Robinson Equation and Its Application to Phase Equilibrium in a System Containing Methanol.” Fluid Phase Equilibria 24, no. 1 (January 1, 1985): 25-41. doi:10.1016/0378-3812(85)87035-7.
- 3
Privat, R., and J.-N. Jaubert. “PPR78, a Thermodynamic Model for the Prediction of Petroleum Fluid-Phase Behaviour,” 11. EDP Sciences, 2011. doi:10.1051/jeep/201100011.
Examples
T-P initialization, and exploring each phase’s properties:
>>> eos = PR(Tc=507.6, Pc=3025000.0, omega=0.2975, T=400., P=1E6) >>> eos.V_l, eos.V_g (0.000156073184785, 0.0021418768167) >>> eos.phase 'l/g' >>> eos.H_dep_l, eos.H_dep_g (-26111.8775716, -3549.30057795) >>> eos.S_dep_l, eos.S_dep_g (-58.098447843, -6.4394518931) >>> eos.U_dep_l, eos.U_dep_g (-22942.1657091, -2365.3923474) >>> eos.G_dep_l, eos.G_dep_g (-2872.49843435, -973.51982071) >>> eos.A_dep_l, eos.A_dep_g (297.21342811, 210.38840980) >>> eos.beta_l, eos.beta_g (0.00269337091778, 0.0101232239111) >>> eos.kappa_l, eos.kappa_g (9.3357215438e-09, 1.97106698097e-06) >>> eos.Cp_minus_Cv_l, eos.Cp_minus_Cv_g (48.510162249, 44.544161128) >>> eos.Cv_dep_l, eos.Cp_dep_l (18.8921126734, 59.0878123050)
P-T initialization, liquid phase, and round robin trip:
>>> eos = PR(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 0.000130222125139, -31134.75084, -72.47561931)
T-V initialization, liquid phase:
>>> eos2 = PR(Tc=507.6, Pc=3025000, omega=0.2975, T=299., V=eos.V_l) >>> eos2.P, eos2.phase (1000000.00, 'l')
P-V initialization at same state:
>>> eos3 = PR(Tc=507.6, Pc=3025000, omega=0.2975, V=eos.V_l, P=1E6) >>> eos3.T, eos3.phase (299.0000000000, 'l')
Methods
P_max_at_V(V)Method to calculate the maximum pressure the EOS can create at a constant volume, if one exists; returns None otherwise.
Method to calculate and its first and second derivatives for this EOS.
a_alpha_pure(T)Method to calculate for this EOS.
Method to calculate the third temperature derivative of a_alpha.
solve_T(P, V[, solution])Method to calculate T from a specified P and V for the PR EOS.
- P_max_at_V(V)[source]¶
Method to calculate the maximum pressure the EOS can create at a constant volume, if one exists; returns None otherwise.
- Parameters
- V
float Constant molar volume, [m^3/mol]
- V
- Returns
- P
float Maximum possible isochoric pressure, [Pa]
- P
Notes
The analytical determination of this formula involved some part of the discriminant, and much black magic.
Examples
>>> e = PR(P=1e5, V=0.0001437, Tc=512.5, Pc=8084000.0, omega=0.559) >>> e.P_max_at_V(e.V) 2247886208.7
- Zc = 0.30740130869870386¶
Mechanical compressibility of Peng-Robinson EOS
- a_alpha_and_derivatives_pure(T)[source]¶
Method to calculate and its first and second derivatives for this EOS. Uses the set values of Tc, kappa, and a.
- Parameters
- T
float Temperature at which to calculate the values, [-]
- T
- Returns
Notes
This method does not alter the object’s state and the temperature provided can be a different than that of the object.
Examples
Dodecane at 250 K:
>>> eos = PR(Tc=658.0, Pc=1820000.0, omega=0.562, T=500., P=1e5) >>> eos.a_alpha_and_derivatives_pure(250.0) (15.66839156301, -0.03094091246957, 9.243186769880e-05)
- a_alpha_pure(T)[source]¶
Method to calculate for this EOS. Uses the set values of Tc, kappa, and a.
- Parameters
- T
float Temperature at which to calculate the value, [-]
- T
- Returns
- a_alpha
float Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- a_alpha
Notes
This method does not alter the object’s state and the temperature provided can be a different than that of the object.
Examples
Dodecane at 250 K:
>>> eos = PR(Tc=658.0, Pc=1820000.0, omega=0.562, T=500., P=1e5) >>> eos.a_alpha_pure(250.0) 15.66839156301
- c1 = 0.4572355289213822¶
Full value of the constant in the a parameter
- c2 = 0.07779607390388846¶
Full value of the constant in the b parameter
- d3a_alpha_dT3_pure(T)[source]¶
Method to calculate the third temperature derivative of a_alpha. Uses the set values of Tc, kappa, and a. This property is not normally needed.
- Parameters
- T
float Temperature at which to calculate the derivative, [-]
- T
- Returns
- d3a_alpha_dT3
float Third temperature derivative of coefficient calculated by EOS-specific method, [J^2/mol^2/Pa/K^3]
- d3a_alpha_dT3
Notes
This method does not alter the object’s state and the temperature provided can be a different than that of the object.
Examples
Dodecane at 500 K:
>>> eos = PR(Tc=658.0, Pc=1820000.0, omega=0.562, T=500., P=1e5) >>> eos.d3a_alpha_dT3_pure(500.0) -9.8038800671e-08
- solve_T(P, V, solution=None)[source]¶
Method to calculate T from a specified P and V for the PR EOS. Uses Tc, a, b, and kappa as well, obtained from the class’s namespace.
- Parameters
- Returns
- T
float Temperature, [K]
- T
Notes
The exact solution can be derived as follows, and is excluded for breviety.
>>> from sympy import * >>> P, T, V = symbols('P, T, V') >>> Tc, Pc, omega = symbols('Tc, Pc, omega') >>> R, a, b, kappa = symbols('R, a, b, kappa') >>> a_alpha = a*(1 + kappa*(1-sqrt(T/Tc)))**2 >>> PR_formula = R*T/(V-b) - a_alpha/(V*(V+b)+b*(V-b)) - P >>> #solve(PR_formula, T)
After careful evaluation of the results of the analytical formula, it was discovered, that numerical precision issues required several NR refinement iterations; at at times, when the analytical value is extremely erroneous, a call to a full numerical solver not using the analytical solution at all is required.
Examples
>>> eos = PR(Tc=658.0, Pc=1820000.0, omega=0.562, T=500., P=1e5) >>> eos.solve_T(P=eos.P, V=eos.V_g) 500.0000000
Peng Robinson (1978)¶
- class thermo.eos.PR78(Tc, Pc, omega, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos.PRClass for solving the Peng-Robinson cubic equation of state for a pure compound according to the 1978 variant [1] [2]. Subclasses
PR, which provides everything except the variable kappa. Solves the EOS on initialization. SeePRfor further documentation.- Parameters
Notes
This variant is recommended over the original.
References
- 1
Robinson, Donald B, and Ding-Yu Peng. The Characterization of the Heptanes and Heavier Fractions for the GPA Peng-Robinson Programs. Tulsa, Okla.: Gas Processors Association, 1978.
- 2
Robinson, Donald B., Ding-Yu Peng, and Samuel Y-K Chung. “The Development of the Peng - Robinson Equation and Its Application to Phase Equilibrium in a System Containing Methanol.” Fluid Phase Equilibria 24, no. 1 (January 1, 1985): 25-41. doi:10.1016/0378-3812(85)87035-7.
Examples
P-T initialization (furfuryl alcohol), liquid phase:
>>> eos = PR78(Tc=632, Pc=5350000, omega=0.734, T=299., P=1E6) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 8.3519628969e-05, -63764.671093, -130.737153225)
- high_omega_constants = (0.379642, 1.48503, -0.164423, 0.016666)¶
Constants for the kappa formula for the high-omega region.
- low_omega_constants = (0.37464, 1.54226, -0.26992)¶
Constants for the kappa formula for the low-omega region.
Peng Robinson Stryjek-Vera¶
- class thermo.eos.PRSV(Tc, Pc, omega, T=None, P=None, V=None, kappa1=None)[source]¶
Bases:
thermo.eos.PRClass for solving the Peng-Robinson-Stryjek-Vera equations of state for a pure compound as given in [1]. The same as the Peng-Robinson EOS, except with a different kappa formula and with an optional fit parameter. Subclasses
PR, which provides only several constants. SeePRfor further documentation and examples.- Parameters
Notes
[1] recommends that kappa1 be set to 0 for Tr > 0.7. This is not done by default; the class boolean kappa1_Tr_limit may be set to True and the problem re-solved with that specified if desired. kappa1_Tr_limit is not supported for P-V inputs.
Solutions for P-V solve for T with SciPy’s newton solver, as there is no analytical solution for T
[2] and [3] are two more resources documenting the PRSV EOS. [4] lists kappa values for 69 additional compounds. See also PRSV2. Note that tabulated kappa values should be used with the critical parameters used in their fits. Both [1] and [4] only considered vapor pressure in fitting the parameter.
References
- 1(1,2,3,4,5)
Stryjek, R., and J. H. Vera. “PRSV: An Improved Peng-Robinson Equation of State for Pure Compounds and Mixtures.” The Canadian Journal of Chemical Engineering 64, no. 2 (April 1, 1986): 323-33. doi:10.1002/cjce.5450640224.
- 2
Stryjek, R., and J. H. Vera. “PRSV - An Improved Peng-Robinson Equation of State with New Mixing Rules for Strongly Nonideal Mixtures.” The Canadian Journal of Chemical Engineering 64, no. 2 (April 1, 1986): 334-40. doi:10.1002/cjce.5450640225.
- 3
Stryjek, R., and J. H. Vera. “Vapor-liquid Equilibrium of Hydrochloric Acid Solutions with the PRSV Equation of State.” Fluid Phase Equilibria 25, no. 3 (January 1, 1986): 279-90. doi:10.1016/0378-3812(86)80004-8.
- 4(1,2)
Proust, P., and J. H. Vera. “PRSV: The Stryjek-Vera Modification of the Peng-Robinson Equation of State. Parameters for Other Pure Compounds of Industrial Interest.” The Canadian Journal of Chemical Engineering 67, no. 1 (February 1, 1989): 170-73. doi:10.1002/cjce.5450670125.
Examples
P-T initialization (hexane, with fit parameter in [1]), liquid phase:
>>> eos = PRSV(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6, kappa1=0.05104) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 0.000130126913554, -31698.926746, -74.16751538)
Methods
Method to calculate and its first and second derivatives for this EOS.
a_alpha_pure(T)Method to calculate for this EOS.
solve_T(P, V[, solution])Method to calculate T from a specified P and V for the PRSV EOS.
- a_alpha_and_derivatives_pure(T)[source]¶
Method to calculate and its first and second derivatives for this EOS. Uses the set values of Tc, kappa0, kappa1, and a.
The a_alpha function is shown below; the first and second derivatives are not shown for brevity.
- Parameters
- T
float Temperature at which to calculate the values, [-]
- T
- Returns
Notes
This method does not alter the object’s state and the temperature provided can be a different than that of the object.
The expressions can be derived as follows:
>>> from sympy import * >>> P, T, V = symbols('P, T, V') >>> Tc, Pc, omega = symbols('Tc, Pc, omega') >>> R, a, b, kappa0, kappa1 = symbols('R, a, b, kappa0, kappa1') >>> kappa = kappa0 + kappa1*(1 + sqrt(T/Tc))*(Rational(7, 10)-T/Tc) >>> a_alpha = a*(1 + kappa*(1-sqrt(T/Tc)))**2 >>> # diff(a_alpha, T) >>> # diff(a_alpha, T, 2)
Examples
>>> eos = PRSV(Tc=507.6, Pc=3025000, omega=0.2975, T=406.08, P=1E6, kappa1=0.05104) >>> eos.a_alpha_and_derivatives_pure(185.0) (4.76865472591, -0.0101408587212, 3.9138298092e-05)
- a_alpha_pure(T)[source]¶
Method to calculate for this EOS. Uses the set values of Tc, kappa0, kappa1, and a.
- Parameters
- T
float Temperature at which to calculate the value, [-]
- T
- Returns
- a_alpha
float Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- a_alpha
Notes
This method does not alter the object’s state and the temperature provided can be a different than that of the object.
Examples
>>> eos = PRSV(Tc=507.6, Pc=3025000, omega=0.2975, T=406.08, P=1E6, kappa1=0.05104) >>> eos.a_alpha_pure(185.0) 4.7686547259
- solve_T(P, V, solution=None)[source]¶
Method to calculate T from a specified P and V for the PRSV EOS. Uses Tc, a, b, kappa0 and kappa as well, obtained from the class’s namespace.
- Parameters
- Returns
- T
float Temperature, [K]
- T
Notes
Not guaranteed to produce a solution. There are actually two solution, one much higher than normally desired; it is possible the solver could converge on this.
Peng Robinson Stryjek-Vera 2¶
- class thermo.eos.PRSV2(Tc, Pc, omega, T=None, P=None, V=None, kappa1=0, kappa2=0, kappa3=0)[source]¶
Bases:
thermo.eos.PRClass for solving the Peng-Robinson-Stryjek-Vera 2 equations of state for a pure compound as given in [1]. The same as the Peng-Robinson EOS, except with a different kappa formula and with three fit parameters. Subclasses
PR, which provides only several constants. SeePRfor further documentation and examples.- Parameters
- Tc
float Critical temperature, [K]
- Pc
float Critical pressure, [Pa]
- omega
float Acentric factor, [-]
- T
float,optional Temperature, [K]
- P
float,optional Pressure, [Pa]
- V
float,optional Molar volume, [m^3/mol]
- kappa1
float,optional Fit parameter; available in [1] for over 90 compounds, [-]
- kappa2
float,optional Fit parameter; available in [1] for over 90 compounds, [-]
- kappa
float,optional Fit parameter; available in [1] for over 90 compounds, [-]
- Tc
Notes
Note that tabulated kappa values should be used with the critical parameters used in their fits. [1] considered only vapor pressure in fitting the parameter.
References
- 1(1,2,3,4,5,6)
Stryjek, R., and J. H. Vera. “PRSV2: A Cubic Equation of State for Accurate Vapor-liquid Equilibria Calculations.” The Canadian Journal of Chemical Engineering 64, no. 5 (October 1, 1986): 820-26. doi:10.1002/cjce.5450640516.
Examples
P-T initialization (hexane, with fit parameter in [1]), liquid phase:
>>> eos = PRSV2(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6, kappa1=0.05104, kappa2=0.8634, kappa3=0.460) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 0.000130188257591, -31496.1841687, -73.615282963)
Methods
Method to calculate and its first and second derivatives for this EOS.
a_alpha_pure(T)Method to calculate for this EOS.
solve_T(P, V[, solution])Method to calculate T from a specified P and V for the PRSV2 EOS.
- a_alpha_and_derivatives_pure(T)[source]¶
Method to calculate and its first and second derivatives for this EOS. Uses the set values of Tc, kappa0, kappa1, kappa2, kappa3, and a.
- Parameters
- T
float Temperature at which to calculate the values, [-]
- T
- Returns
Notes
The first and second derivatives of a_alpha are available through the following SymPy expression.
>>> from sympy import * >>> P, T, V = symbols('P, T, V') >>> Tc, Pc, omega = symbols('Tc, Pc, omega') >>> R, a, b, kappa0, kappa1, kappa2, kappa3 = symbols('R, a, b, kappa0, kappa1, kappa2, kappa3') >>> Tr = T/Tc >>> kappa = kappa0 + (kappa1 + kappa2*(kappa3-Tr)*(1-sqrt(Tr)))*(1+sqrt(Tr))*(Rational('0.7')-Tr) >>> a_alpha = a*(1 + kappa*(1-sqrt(T/Tc)))**2 >>> diff(a_alpha, T) >>> diff(a_alpha, T, 2)
Examples
>>> eos = PRSV2(Tc=507.6, Pc=3025000, omega=0.2975, T=400., P=1E6, kappa1=0.05104, kappa2=0.8634, kappa3=0.460) >>> eos.a_alpha_and_derivatives_pure(311.0) (3.7245418495, -0.0066115440470, 2.05871011677e-05)
- a_alpha_pure(T)[source]¶
Method to calculate for this EOS. Uses the set values of Tc, kappa0, kappa1, kappa2, kappa3, and a.
- Parameters
- T
float Temperature at which to calculate the values, [-]
- T
- Returns
- a_alpha
float Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- a_alpha
Examples
>>> eos = PRSV2(Tc=507.6, Pc=3025000, omega=0.2975, T=400., P=1E6, kappa1=0.05104, kappa2=0.8634, kappa3=0.460) >>> eos.a_alpha_pure(1276.0) 33.321674050
- solve_T(P, V, solution=None)[source]¶
Method to calculate T from a specified P and V for the PRSV2 EOS. Uses Tc, a, b, kappa0, kappa1, kappa2, and kappa3 as well, obtained from the class’s namespace.
- Parameters
- Returns
- T
float Temperature, [K]
- T
Notes
Not guaranteed to produce a solution. There are actually 8 solutions, six with an imaginary component at a tested point. The two temperature solutions are quite far apart, with one much higher than the other; it is possible the solver could converge on the higher solution, so use T inputs with care. This extra solution is a perfectly valid one however. The secant method is implemented at present.
Examples
>>> eos = PRSV2(Tc=507.6, Pc=3025000, omega=0.2975, T=400., P=1E6, kappa1=0.05104, kappa2=0.8634, kappa3=0.460) >>> eos.solve_T(P=eos.P, V=eos.V_g) 400.0
Peng Robinson Twu (1995)¶
- class thermo.eos.TWUPR(Tc, Pc, omega, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos_alpha_functions.TwuPR95_a_alpha,thermo.eos.PRClass for solving the Twu (1995) [1] variant of the Peng-Robinson cubic equation of state for a pure compound. Subclasses
PR, which provides the methods for solving the EOS and calculating its assorted relevant thermodynamic properties. Solves the EOS on initialization.The main implemented method here is
a_alpha_and_derivatives_pure, which sets and its first and second derivatives.Two of T, P, and V are needed to solve the EOS.
For sub-critical conditions:
L0, M0, N0 = 0.125283, 0.911807, 1.948150;
L1, M1, N1 = 0.511614, 0.784054, 2.812520
For supercritical conditions:
L0, M0, N0 = 0.401219, 4.963070, -0.2;
L1, M1, N1 = 0.024955, 1.248089, -8.
- Parameters
Notes
Claimed to be more accurate than the PR, PR78 and PRSV equations.
There is no analytical solution for T. There are multiple possible solutions for T under certain conditions; no guaranteed are provided regarding which solution is obtained.
References
- 1
Twu, Chorng H., John E. Coon, and John R. Cunningham. “A New Generalized Alpha Function for a Cubic Equation of State Part 1. Peng-Robinson Equation.” Fluid Phase Equilibria 105, no. 1 (March 15, 1995): 49-59. doi:10.1016/0378-3812(94)02601-V.
Examples
>>> eos = TWUPR(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6) >>> eos.V_l, eos.H_dep_l, eos.S_dep_l (0.00013017554170, -31652.73712, -74.112850429)
Methods
Method to calculate and its first and second derivatives for the Twu alpha function.
a_alpha_pure(T)Method to calculate for the Twu alpha function.
- a_alpha_and_derivatives_pure(T)¶
Method to calculate and its first and second derivatives for the Twu alpha function. Uses the set values of Tc, omega and a.
For sub-critical conditions:
L0, M0, N0 = 0.125283, 0.911807, 1.948150;
L1, M1, N1 = 0.511614, 0.784054, 2.812520
For supercritical conditions:
L0, M0, N0 = 0.401219, 4.963070, -0.2;
L1, M1, N1 = 0.024955, 1.248089, -8.
- Parameters
- T
float Temperature at which to calculate the values, [-]
- T
- Returns
Notes
This method does not alter the object’s state and the temperature provided can be a different than that of the object.
The derivatives are somewhat long and are not described here for brevity; they are obtainable from the following SymPy expression.
>>> from sympy import * >>> T, Tc, omega, N1, N0, M1, M0, L1, L0 = symbols('T, Tc, omega, N1, N0, M1, M0, L1, L0') >>> Tr = T/Tc >>> alpha0 = Tr**(N0*(M0-1))*exp(L0*(1-Tr**(N0*M0))) >>> alpha1 = Tr**(N1*(M1-1))*exp(L1*(1-Tr**(N1*M1))) >>> alpha = alpha0 + omega*(alpha1-alpha0) >>> diff(alpha, T) >>> diff(alpha, T, T)
- a_alpha_pure(T)¶
Method to calculate for the Twu alpha function. Uses the set values of Tc, omega and a.
For sub-critical conditions:
L0, M0, N0 = 0.125283, 0.911807, 1.948150;
L1, M1, N1 = 0.511614, 0.784054, 2.812520
For supercritical conditions:
L0, M0, N0 = 0.401219, 4.963070, -0.2;
L1, M1, N1 = 0.024955, 1.248089, -8.
- Parameters
- T
float Temperature at which to calculate the value, [-]
- T
- Returns
- a_alpha
float Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- a_alpha
Notes
This method does not alter the object’s state and the temperature provided can be a different than that of the object.
Peng Robinson Polynomial alpha Function¶
- class thermo.eos.PRTranslatedPoly(Tc, Pc, omega, alpha_coeffs=None, c=0.0, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos_alpha_functions.Poly_a_alpha,thermo.eos.PRTranslatedClass for solving the volume translated Peng-Robinson equation of state with a polynomial alpha function. With the right coefficients, this model can reproduce any property incredibly well. Subclasses
PRTranslated. Solves the EOS on initialization. This is intended as a base class for all translated variants of the Peng-Robinson EOS.- Parameters
- Tc
float Critical temperature, [K]
- Pc
float Critical pressure, [Pa]
- omega
float Acentric factor, [-]
- alpha_coeffs
tupleorNone Coefficients which may be specified by subclasses; set to None to use the original Peng-Robinson alpha function, [-]
- c
float,optional Volume translation parameter, [m^3/mol]
- T
float,optional Temperature, [K]
- P
float,optional Pressure, [Pa]
- V
float,optional Molar volume, [m^3/mol]
- Tc
Examples
Methanol, with alpha functions reproducing CoolProp’s implementation of its vapor pressure (up to 13 coefficients)
>>> alpha_coeffs_exact = [9.645280470011588e-32, -4.362226651748652e-28, 9.034194757823037e-25, -1.1343330204981244e-21, 9.632898335494218e-19, -5.841502902171077e-16, 2.601801729901228e-13, -8.615431349241052e-11, 2.1202999753932622e-08, -3.829144045293198e-06, 0.0004930777289075716, -0.04285337965522619, 2.2473964123842705, -51.13852710672087] >>> kwargs = dict(Tc=512.5, Pc=8084000.0, omega=0.559, alpha_coeffs=alpha_coeffs_exact, c=1.557458e-05) >>> eos = PRTranslatedPoly(T=300, P=1e5, **kwargs) >>> eos.Psat(500)/PropsSI("P", 'T', 500.0, 'Q', 0, 'methanol') 1.0000112765
Methods
Method to calculate a_alpha and its first and second derivatives given that there is a polynomial equation for .
a_alpha_pure(T)Method to calculate a_alpha given that there is a polynomial equation for .
- a_alpha_and_derivatives_pure(T)¶
Method to calculate a_alpha and its first and second derivatives given that there is a polynomial equation for .
- Parameters
- T
float Temperature, [K]
- T
- Returns
- a_alphas
list[float] Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- da_alpha_dTs
list[float] Temperature derivative of coefficient calculated by EOS-specific method, [J^2/mol^2/Pa/K]
- d2a_alpha_dT2s
list[float] Second temperature derivative of coefficient calculated by EOS-specific method, [J^2/mol^2/Pa/K**2]
- a_alphas
Volume Translated Peng-Robinson Family EOSs¶
Peng Robinson Translated¶
- class thermo.eos.PRTranslated(Tc, Pc, omega, alpha_coeffs=None, c=0.0, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos.PRClass for solving the volume translated Peng-Robinson equation of state. Subclasses
PR. Solves the EOS on initialization. This is intended as a base class for all translated variants of the Peng-Robinson EOS.- Parameters
- Tc
float Critical temperature, [K]
- Pc
float Critical pressure, [Pa]
- omega
float Acentric factor, [-]
- alpha_coeffs
tupleorNone Coefficients which may be specified by subclasses; set to None to use the original Peng-Robinson alpha function, [-]
- c
float,optional Volume translation parameter, [m^3/mol]
- T
float,optional Temperature, [K]
- P
float,optional Pressure, [Pa]
- V
float,optional Molar volume, [m^3/mol]
- Tc
References
- 1
Gmehling, Jürgen, Michael Kleiber, Bärbel Kolbe, and Jürgen Rarey. Chemical Thermodynamics for Process Simulation. John Wiley & Sons, 2019.
Examples
P-T initialization:
>>> eos = PRTranslated(T=305, P=1.1e5, Tc=512.5, Pc=8084000.0, omega=0.559, c=-1e-6) >>> eos.phase, eos.V_l, eos.V_g ('l/g', 4.90798083711e-05, 0.0224350982488)
Peng Robinson Translated Twu (1991)¶
- class thermo.eos.PRTranslatedTwu(Tc, Pc, omega, alpha_coeffs=None, c=0.0, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos_alpha_functions.Twu91_a_alpha,thermo.eos.PRTranslatedClass for solving the volume translated Peng-Robinson equation of state with the Twu (1991) [1] alpha function. Subclasses
thermo.eos_alpha_functions.Twu91_a_alphaandPRTranslated. Solves the EOS on initialization.- Parameters
- Tc
float Critical temperature, [K]
- Pc
float Critical pressure, [Pa]
- omega
float Acentric factor, [-]
- alpha_coeffs
tuple(float[3]) Coefficients L, M, N (also called C1, C2, C3) of TWU 1991 form, [-]
- c
float,optional Volume translation parameter, [m^3/mol]
- T
float,optional Temperature, [K]
- P
float,optional Pressure, [Pa]
- V
float,optional Molar volume, [m^3/mol]
- Tc
Notes
This variant offers substantial improvements to the PR-type EOSs - likely getting about as accurate as this form of cubic equation can get.
References
- 1
Twu, Chorng H., David Bluck, John R. Cunningham, and John E. Coon. “A Cubic Equation of State with a New Alpha Function and a New Mixing Rule.” Fluid Phase Equilibria 69 (December 10, 1991): 33-50. doi:10.1016/0378-3812(91)90024-2.
Examples
P-T initialization:
>>> alpha_coeffs = (0.694911381318495, 0.919907783415812, 1.70412689631515) >>> kwargs = dict(Tc=512.5, Pc=8084000.0, omega=0.559, alpha_coeffs=alpha_coeffs, c=-1e-6) >>> eos = PRTranslatedTwu(T=300, P=1e5, **kwargs) >>> eos.phase, eos.V_l, eos.V_g ('l/g', 4.8918748906e-05, 0.024314406330)
Peng Robinson Translated-Consistent¶
- class thermo.eos.PRTranslatedConsistent(Tc, Pc, omega, alpha_coeffs=None, c=None, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos.PRTranslatedTwuClass for solving the volume translated Le Guennec, Privat, and Jaubert revision of the Peng-Robinson equation of state for a pure compound according to [1]. Subclasses
PRTranslatedTwu, which provides everything except the estimation of c and the alpha coefficients. This model’s alpha is based on the TWU 1991 model; when estimating, N is set to 2. Solves the EOS on initialization. SeePRTranslatedfor further documentation.If c is not provided, it is estimated as:
If alpha_coeffs is not provided, the parameters L and M are estimated from the acentric factor as follows:
- Parameters
- Tc
float Critical temperature, [K]
- Pc
float Critical pressure, [Pa]
- omega
float Acentric factor, [-]
- alpha_coeffs
tuple(float[3]),optional Coefficients L, M, N (also called C1, C2, C3) of TWU 1991 form, [-]
- c
float,optional Volume translation parameter, [m^3/mol]
- T
float,optional Temperature, [K]
- P
float,optional Pressure, [Pa]
- V
float,optional Molar volume, [m^3/mol]
- Tc
Notes
This variant offers substantial improvements to the PR-type EOSs - likely getting about as accurate as this form of cubic equation can get.
References
- 1
Le Guennec, Yohann, Romain Privat, and Jean-Noël Jaubert. “Development of the Translated-Consistent Tc-PR and Tc-RK Cubic Equations of State for a Safe and Accurate Prediction of Volumetric, Energetic and Saturation Properties of Pure Compounds in the Sub- and Super-Critical Domains.” Fluid Phase Equilibria 429 (December 15, 2016): 301-12. https://doi.org/10.1016/j.fluid.2016.09.003.
Examples
P-T initialization (methanol), liquid phase:
>>> eos = PRTranslatedConsistent(Tc=507.6, Pc=3025000, omega=0.2975, T=250., P=1E6) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 0.000124374813374486, -34155.16119794619, -83.34913258614345)
Peng Robinson Translated (Pina-Martinez, Privat, and Jaubert Variant)¶
- class thermo.eos.PRTranslatedPPJP(Tc, Pc, omega, c=0.0, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos.PRTranslatedClass for solving the volume translated Pina-Martinez, Privat, Jaubert, and Peng revision of the Peng-Robinson equation of state for a pure compound according to [1]. Subclasses
PRTranslated, which provides everything except the variable kappa. Solves the EOS on initialization. SeePRTranslatedfor further documentation.- Parameters
Notes
This variant offers incremental improvements in accuracy only, but those can be fairly substantial for some substances.
References
- 1
Pina-Martinez, Andrés, Romain Privat, Jean-Noël Jaubert, and Ding-Yu Peng. “Updated Versions of the Generalized Soave a-Function Suitable for the Redlich-Kwong and Peng-Robinson Equations of State.” Fluid Phase Equilibria, December 7, 2018. https://doi.org/10.1016/j.fluid.2018.12.007.
Examples
P-T initialization (methanol), liquid phase:
>>> eos = PRTranslatedPPJP(Tc=507.6, Pc=3025000, omega=0.2975, c=0.6390E-6, T=250., P=1E6) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 0.0001229231238092, -33466.2428296, -80.75610242427)
Soave-Redlich-Kwong Family EOSs¶
Standard SRK¶
- class thermo.eos.SRK(Tc, Pc, omega, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos.GCEOSClass for solving the Soave-Redlich-Kwong [1] [2] [3] cubic equation of state for a pure compound. Subclasses
GCEOS, which provides the methods for solving the EOS and calculating its assorted relevant thermodynamic properties. Solves the EOS on initialization.Two of T, P, and V are needed to solve the EOS.
- Parameters
References
- 1
Soave, Giorgio. “Equilibrium Constants from a Modified Redlich-Kwong Equation of State.” Chemical Engineering Science 27, no. 6 (June 1972): 1197-1203. doi:10.1016/0009-2509(72)80096-4.
- 2
Poling, Bruce E. The Properties of Gases and Liquids. 5th edition. New York: McGraw-Hill Professional, 2000.
- 3
Walas, Stanley M. Phase Equilibria in Chemical Engineering. Butterworth-Heinemann, 1985.
Examples
>>> eos = SRK(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 0.000146821077354, -31754.663859, -74.373272044)
Methods
P_max_at_V(V)Method to calculate the maximum pressure the EOS can create at a constant volume, if one exists; returns None otherwise.
Method to calculate and its first and second derivatives for this EOS.
a_alpha_pure(T)Method to calculate for this EOS.
solve_T(P, V[, solution])Method to calculate T from a specified P and V for the SRK EOS.
- P_max_at_V(V)[source]¶
Method to calculate the maximum pressure the EOS can create at a constant volume, if one exists; returns None otherwise.
- Parameters
- V
float Constant molar volume, [m^3/mol]
- V
- Returns
- P
float Maximum possible isochoric pressure, [Pa]
- P
Notes
The analytical determination of this formula involved some part of the discriminant, and much black magic.
Examples
>>> e = SRK(P=1e5, V=0.0001437, Tc=512.5, Pc=8084000.0, omega=0.559) >>> e.P_max_at_V(e.V) 490523786.2
- a_alpha_and_derivatives_pure(T)[source]¶
Method to calculate and its first and second derivatives for this EOS. Uses the set values of Tc, m, and a.
- Parameters
- T
float Temperature at which to calculate the values, [-]
- T
- Returns
- c1 = 0.4274802335403414¶
Full value of the constant in the a parameter
- c2 = 0.08664034996495772¶
Full value of the constant in the b parameter
- solve_T(P, V, solution=None)[source]¶
Method to calculate T from a specified P and V for the SRK EOS. Uses a, b, and Tc obtained from the class’s namespace.
- Parameters
- Returns
- T
float Temperature, [K]
- T
Notes
The exact solution can be derived as follows; it is excluded for breviety.
>>> from sympy import * >>> P, T, V, R, a, b, m = symbols('P, T, V, R, a, b, m') >>> Tc, Pc, omega = symbols('Tc, Pc, omega') >>> a_alpha = a*(1 + m*(1-sqrt(T/Tc)))**2 >>> SRK = R*T/(V-b) - a_alpha/(V*(V+b)) - P >>> solve(SRK, T)
Twu SRK (1995)¶
- class thermo.eos.TWUSRK(Tc, Pc, omega, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos_alpha_functions.TwuSRK95_a_alpha,thermo.eos.SRKClass for solving the Soave-Redlich-Kwong cubic equation of state for a pure compound. Subclasses
GCEOS, which provides the methods for solving the EOS and calculating its assorted relevant thermodynamic properties. Solves the EOS on initialization.The main implemented method here is
a_alpha_and_derivatives_pure, which sets and its first and second derivatives.Two of T, P, and V are needed to solve the EOS.
For sub-critical conditions:
L0, M0, N0 = 0.141599, 0.919422, 2.496441
L1, M1, N1 = 0.500315, 0.799457, 3.291790
For supercritical conditions:
L0, M0, N0 = 0.441411, 6.500018, -0.20
L1, M1, N1 = 0.032580, 1.289098, -8.0
- Parameters
Notes
There is no analytical solution for T. There are multiple possible solutions for T under certain conditions; no guaranteed are provided regarding which solution is obtained.
References
- 1
Twu, Chorng H., John E. Coon, and John R. Cunningham. “A New Generalized Alpha Function for a Cubic Equation of State Part 2. Redlich-Kwong Equation.” Fluid Phase Equilibria 105, no. 1 (March 15, 1995): 61-69. doi:10.1016/0378-3812(94)02602-W.
Examples
>>> eos = TWUSRK(Tc=507.6, Pc=3025000, omega=0.2975, T=299., P=1E6) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 0.000146892222966, -31612.6025870, -74.022966093)
Methods
Method to calculate and its first and second derivatives for the Twu alpha function.
a_alpha_pure(T)Method to calculate for the Twu alpha function.
- a_alpha_and_derivatives_pure(T)¶
Method to calculate and its first and second derivatives for the Twu alpha function. Uses the set values of Tc, omega and a.
For sub-critical conditions:
L0, M0, N0 = 0.141599, 0.919422, 2.496441
L1, M1, N1 = 0.500315, 0.799457, 3.291790
For supercritical conditions:
L0, M0, N0 = 0.441411, 6.500018, -0.20
L1, M1, N1 = 0.032580, 1.289098, -8.0
- Parameters
- T
float Temperature at which to calculate the values, [-]
- T
- Returns
Notes
This method does not alter the object’s state and the temperature provided can be a different than that of the object.
The derivatives are somewhat long and are not described here for brevity; they are obtainable from the following SymPy expression.
>>> from sympy import * >>> T, Tc, omega, N1, N0, M1, M0, L1, L0 = symbols('T, Tc, omega, N1, N0, M1, M0, L1, L0') >>> Tr = T/Tc >>> alpha0 = Tr**(N0*(M0-1))*exp(L0*(1-Tr**(N0*M0))) >>> alpha1 = Tr**(N1*(M1-1))*exp(L1*(1-Tr**(N1*M1))) >>> alpha = alpha0 + omega*(alpha1-alpha0) >>> diff(alpha, T) >>> diff(alpha, T, T)
- a_alpha_pure(T)¶
Method to calculate for the Twu alpha function. Uses the set values of Tc, omega and a.
For sub-critical conditions:
L0, M0, N0 = 0.141599, 0.919422, 2.496441
L1, M1, N1 = 0.500315, 0.799457, 3.291790
For supercritical conditions:
L0, M0, N0 = 0.441411, 6.500018, -0.20
L1, M1, N1 = 0.032580, 1.289098, -8.0
- Parameters
- T
float Temperature at which to calculate the value, [-]
- T
- Returns
- a_alpha
float Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- a_alpha
Notes
This method does not alter the object’s state and the temperature provided can be a different than that of the object.
API SRK¶
- class thermo.eos.APISRK(Tc, Pc, omega=None, T=None, P=None, V=None, S1=None, S2=0)[source]¶
Bases:
thermo.eos.SRKClass for solving the Refinery Soave-Redlich-Kwong cubic equation of state for a pure compound shown in the API Databook [1]. Subclasses
GCEOS, which provides the methods for solving the EOS and calculating its assorted relevant thermodynamic properties. Solves the EOS on initialization.Implemented methods here are a_alpha_and_derivatives, which sets and its first and second derivatives, and solve_T, which from a specified P and V obtains T. Two fit constants are used in this expresion, with an estimation scheme for the first if unavailable and the second may be set to zero.
Two of T, P, and V are needed to solve the EOS.
- Parameters
- Tc
float Critical temperature, [K]
- Pc
float Critical pressure, [Pa]
- omega
float,optional Acentric factor, [-]
- T
float,optional Temperature, [K]
- P
float,optional Pressure, [Pa]
- V
float,optional Molar volume, [m^3/mol]
- S1
float,optional Fit constant or estimated from acentric factor if not provided [-]
- S2
float,optional Fit constant or 0 if not provided [-]
- Tc
References
- 1
API Technical Data Book: General Properties & Characterization. American Petroleum Institute, 7E, 2005.
Examples
>>> eos = APISRK(Tc=514.0, Pc=6137000.0, S1=1.678665, S2=-0.216396, P=1E6, T=299) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 7.0456950702e-05, -42826.286146, -103.626979037)
Methods
Method to calculate and its first and second derivatives for this EOS.
a_alpha_pure(T)Method to calculate for this EOS.
solve_T(P, V[, solution])Method to calculate T from a specified P and V for the API SRK EOS.
- a_alpha_and_derivatives_pure(T)[source]¶
Method to calculate and its first and second derivatives for this EOS. Returns a_alpha, da_alpha_dT, and d2a_alpha_dT2. See GCEOS.a_alpha_and_derivatives for more documentation. Uses the set values of Tc, a, S1, and S2.
- solve_T(P, V, solution=None)[source]¶
Method to calculate T from a specified P and V for the API SRK EOS. Uses a, b, and Tc obtained from the class’s namespace.
- Parameters
- Returns
- T
float Temperature, [K]
- T
Notes
If S2 is set to 0, the solution is the same as in the SRK EOS, and that is used. Otherwise, newton’s method must be used to solve for T. There are 8 roots of T in that case, six of them real. No guarantee can be made regarding which root will be obtained.
SRK Translated¶
- class thermo.eos.SRKTranslated(Tc, Pc, omega, alpha_coeffs=None, c=0.0, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos.SRKClass for solving the volume translated Peng-Robinson equation of state. Subclasses
SRK. Solves the EOS on initialization. This is intended as a base class for all translated variants of the SRK EOS.- Parameters
- Tc
float Critical temperature, [K]
- Pc
float Critical pressure, [Pa]
- omega
float Acentric factor, [-]
- alpha_coeffs
tupleorNone Coefficients which may be specified by subclasses; set to None to use the original Peng-Robinson alpha function, [-]
- c
float,optional Volume translation parameter, [m^3/mol]
- T
float,optional Temperature, [K]
- P
float,optional Pressure, [Pa]
- V
float,optional Molar volume, [m^3/mol]
- Tc
References
- 1
Gmehling, Jürgen, Michael Kleiber, Bärbel Kolbe, and Jürgen Rarey. Chemical Thermodynamics for Process Simulation. John Wiley & Sons, 2019.
Examples
P-T initialization:
>>> eos = SRKTranslated(T=305, P=1.1e5, Tc=512.5, Pc=8084000.0, omega=0.559, c=-1e-6) >>> eos.phase, eos.V_l, eos.V_g ('l/g', 5.5131657318e-05, 0.022447661363)
SRK Translated-Consistent¶
- class thermo.eos.SRKTranslatedConsistent(Tc, Pc, omega, alpha_coeffs=None, c=None, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos_alpha_functions.Twu91_a_alpha,thermo.eos.SRKTranslatedClass for solving the volume translated Le Guennec, Privat, and Jaubert revision of the SRK equation of state for a pure compound according to [1].
This model’s alpha is based on the TWU 1991 model; when estimating, N is set to 2. Solves the EOS on initialization. See SRK for further documentation.
If c is not provided, it is estimated as:
If alpha_coeffs is not provided, the parameters L and M are estimated from the acentric factor as follows:
- Parameters
- Tc
float Critical temperature, [K]
- Pc
float Critical pressure, [Pa]
- omega
float Acentric factor, [-]
- alpha_coeffs
tuple(float[3]),optional Coefficients L, M, N (also called C1, C2, C3) of TWU 1991 form, [-]
- c
float,optional Volume translation parameter, [m^3/mol]
- T
float,optional Temperature, [K]
- P
float,optional Pressure, [Pa]
- V
float,optional Molar volume, [m^3/mol]
- Tc
Notes
This variant offers substantial improvements to the SRK-type EOSs - likely getting about as accurate as this form of cubic equation can get.
References
- 1
Le Guennec, Yohann, Romain Privat, and Jean-Noël Jaubert. “Development of the Translated-Consistent Tc-PR and Tc-RK Cubic Equations of State for a Safe and Accurate Prediction of Volumetric, Energetic and Saturation Properties of Pure Compounds in the Sub- and Super-Critical Domains.” Fluid Phase Equilibria 429 (December 15, 2016): 301-12. https://doi.org/10.1016/j.fluid.2016.09.003.
Examples
P-T initialization (methanol), liquid phase:
>>> eos = SRKTranslatedConsistent(Tc=507.6, Pc=3025000, omega=0.2975, T=250., P=1E6) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 0.00011846802568940222, -34324.05211005662, -83.83861726864234)
SRK Translated (Pina-Martinez, Privat, and Jaubert Variant)¶
- class thermo.eos.SRKTranslatedPPJP(Tc, Pc, omega, c=0.0, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos.SRKClass for solving the volume translated Pina-Martinez, Privat, Jaubert, and Peng revision of the Soave-Redlich-Kwong equation of state for a pure compound according to [1]. Subclasses SRK, which provides everything except the variable kappa. Solves the EOS on initialization. See SRK for further documentation.
- Parameters
Notes
This variant offers incremental improvements in accuracy only, but those can be fairly substantial for some substances.
References
- 1
Pina-Martinez, Andrés, Romain Privat, Jean-Noël Jaubert, and Ding-Yu Peng. “Updated Versions of the Generalized Soave a-Function Suitable for the Redlich-Kwong and Peng-Robinson Equations of State.” Fluid Phase Equilibria, December 7, 2018. https://doi.org/10.1016/j.fluid.2018.12.007.
Examples
P-T initialization (hexane), liquid phase:
>>> eos = SRKTranslatedPPJP(Tc=507.6, Pc=3025000, omega=0.2975, c=22.3098E-6, T=250., P=1E6) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 0.00011666322408111662, -34158.934132722185, -83.06507748137201)
MSRK Translated¶
- class thermo.eos.MSRKTranslated(Tc, Pc, omega, M=None, N=None, alpha_coeffs=None, c=0.0, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos_alpha_functions.Soave_1979_a_alpha,thermo.eos.SRKTranslatedClass for solving the volume translated Soave (1980) alpha function, revision of the Soave-Redlich-Kwong equation of state for a pure compound according to [1]. Uses two fitting parameters N and M to more accurately fit the vapor pressure of pure species. Subclasses SRKTranslated. Solves the EOS on initialization. See SRKTranslated for further documentation.
- Parameters
- Tc
float Critical temperature, [K]
- Pc
float Critical pressure, [Pa]
- omega
float Acentric factor, [-]
- c
float,optional Volume translation parameter, [m^3/mol]
- alpha_coeffs
tuple(float[3]),optional Coefficients M, N of this EOS’s alpha function, [-]
- T
float,optional Temperature, [K]
- P
float,optional Pressure, [Pa]
- V
float,optional Molar volume, [m^3/mol]
- Tc
Notes
This is an older correlation that offers lower accuracy on many properties which were sacrificed to obtain the vapor pressure accuracy. The alpha function of this EOS does not meet any of the consistency requriements for alpha functions.
Coefficients can be found in [2], or estimated with the method in [3]. The estimation method in [3] works as follows, using the acentric factor and true critical compressibility:
An alternate estimation scheme is provided in [1], which provides analytical solutions to calculate the parameters M and N from two points on the vapor pressure curve, suggested as 10 mmHg and 1 atm. This is used as an estimation method here if the parameters are not provided, and the two vapor pressure points are obtained from the original SRK equation of state.
References
- 1(1,2)
Soave, G. “Rigorous and Simplified Procedures for Determining the Pure-Component Parameters in the Redlich—Kwong—Soave Equation of State.” Chemical Engineering Science 35, no. 8 (January 1, 1980): 1725-30. https://doi.org/10.1016/0009-2509(80)85007-X.
- 2
Sandarusi, Jamal A., Arthur J. Kidnay, and Victor F. Yesavage. “Compilation of Parameters for a Polar Fluid Soave-Redlich-Kwong Equation of State.” Industrial & Engineering Chemistry Process Design and Development 25, no. 4 (October 1, 1986): 957-63. https://doi.org/10.1021/i200035a020.
- 3(1,2)
Valderrama, Jose O., Héctor De la Puente, and Ahmed A. Ibrahim. “Generalization of a Polar-Fluid Soave-Redlich-Kwong Equation of State.” Fluid Phase Equilibria 93 (February 11, 1994): 377-83. https://doi.org/10.1016/0378-3812(94)87021-7.
Examples
P-T initialization (hexane), liquid phase:
>>> eos = MSRKTranslated(Tc=507.6, Pc=3025000, omega=0.2975, c=22.0561E-6, M=0.7446, N=0.2476, T=250., P=1E6) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 0.0001169276461322, -34571.6862673, -84.757900348)
Methods
estimate_MN(Tc, Pc, omega[, c])Calculate the alpha values for the MSRK equation to match two pressure points, and solve analytically for the M, N required to match exactly that.
- static estimate_MN(Tc, Pc, omega, c=0.0)[source]¶
Calculate the alpha values for the MSRK equation to match two pressure points, and solve analytically for the M, N required to match exactly that. Since no experimental data is available, make it up with the original SRK EOS.
- Parameters
- Returns
Examples
>>> from sympy import * >>> Tc, m, n = symbols('Tc, m, n') >>> T0, T1 = symbols('T_10, T_760') >>> alpha0, alpha1 = symbols('alpha_10, alpha_760') >>> Eqs = [Eq(alpha0, 1 + (1 - T0/Tc)*(m + n/(T0/Tc))), Eq(alpha1, 1 + (1 - T1/Tc)*(m + n/(T1/Tc)))] >>> solve(Eqs, [n, m])
Van der Waals Equations of State¶
- class thermo.eos.VDW(Tc, Pc, T=None, P=None, V=None, omega=None)[source]¶
Bases:
thermo.eos.GCEOSClass for solving the Van der Waals [1] [2] cubic equation of state for a pure compound. Subclasses
GCEOS, which provides the methods for solving the EOS and calculating its assorted relevant thermodynamic properties. Solves the EOS on initialization.Two of T, P, and V are needed to solve the EOS.
- Parameters
Notes
omega is allowed as an input for compatibility with the other EOS forms, but is not used.
References
- 1
Poling, Bruce E. The Properties of Gases and Liquids. 5th edition. New York: McGraw-Hill Professional, 2000.
- 2
Walas, Stanley M. Phase Equilibria in Chemical Engineering. Butterworth-Heinemann, 1985.
Examples
>>> eos = VDW(Tc=507.6, Pc=3025000, T=299., P=1E6) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 0.000223329856081, -13385.7273746, -32.65923125)
- Attributes
- omega
Methods
P_discriminant_zeros_analytical(T, b, delta, ...)Method to calculate the pressures which zero the discriminant function of the
VDWeos.T_discriminant_zeros_analytical([valid])Method to calculate the temperatures which zero the discriminant function of the
VDWeos.Method to calculate and its first and second derivatives for this EOS.
a_alpha_pure(T)Method to calculate .
solve_T(P, V[, solution])Method to calculate T from a specified P and V for the
VDWEOS.- static P_discriminant_zeros_analytical(T, b, delta, epsilon, a_alpha, valid=False)[source]¶
Method to calculate the pressures which zero the discriminant function of the
VDWeos. This is an cubic function solved analytically.- Parameters
- T
float Temperature, [K]
- b
float Coefficient calculated by EOS-specific method, [m^3/mol]
- delta
float Coefficient calculated by EOS-specific method, [m^3/mol]
- epsilon
float Coefficient calculated by EOS-specific method, [m^6/mol^2]
- a_alpha
float Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- validbool
Whether to filter the calculated pressures so that they are all real, and positive only, [-]
- T
- Returns
Notes
Calculated analytically. Derived as follows. Has multiple solutions.
>>> from sympy import * >>> P, T, V, R, b, a = symbols('P, T, V, R, b, a') >>> P_vdw = R*T/(V-b) - a/(V*V) >>> delta, epsilon = 0, 0 >>> eta = b >>> B = b*P/(R*T) >>> deltas = delta*P/(R*T) >>> thetas = a*P/(R*T)**2 >>> epsilons = epsilon*(P/(R*T))**2 >>> etas = eta*P/(R*T) >>> a_coeff = 1 >>> b_coeff = (deltas - B - 1) >>> c = (thetas + epsilons - deltas*(B+1)) >>> d = -(epsilons*(B+1) + thetas*etas) >>> disc = b_coeff*b_coeff*c*c - 4*a_coeff*c*c*c - 4*b_coeff*b_coeff*b_coeff*d - 27*a_coeff*a_coeff*d*d + 18*a_coeff*b_coeff*c*d >>> base = -(expand(disc/P**2*R**3*T**3/a)) >>> collect(base, P).args
- T_discriminant_zeros_analytical(valid=False)[source]¶
Method to calculate the temperatures which zero the discriminant function of the
VDWeos. This is an analytical cubic function solved analytically.- Parameters
- validbool
Whether to filter the calculated temperatures so that they are all real, and positive only, [-]
- Returns
Notes
Calculated analytically. Derived as follows. Has multiple solutions.
>>> from sympy import * >>> P, T, V, R, b, a = symbols('P, T, V, R, b, a') >>> delta, epsilon = 0, 0 >>> eta = b >>> B = b*P/(R*T) >>> deltas = delta*P/(R*T) >>> thetas = a*P/(R*T)**2 >>> epsilons = epsilon*(P/(R*T))**2 >>> etas = eta*P/(R*T) >>> a_coeff = 1 >>> b_coeff = (deltas - B - 1) >>> c = (thetas + epsilons - deltas*(B+1)) >>> d = -(epsilons*(B+1) + thetas*etas) >>> disc = b_coeff*b_coeff*c*c - 4*a_coeff*c*c*c - 4*b_coeff*b_coeff*b_coeff*d - 27*a_coeff*a_coeff*d*d + 18*a_coeff*b_coeff*c*d >>> base = -(expand(disc/P**2*R**3*T**3/a)) >>> base_T = simplify(base*T**3) >>> sln = collect(expand(base_T), T).args
- a_alpha_and_derivatives_pure(T)[source]¶
Method to calculate and its first and second derivatives for this EOS. Uses the set values of a.
- Parameters
- T
float Temperature at which to calculate the values, [-]
- T
- Returns
Redlich-Kwong Equations of State¶
- class thermo.eos.RK(Tc, Pc, T=None, P=None, V=None, omega=None)[source]¶
Bases:
thermo.eos.GCEOSClass for solving the Redlich-Kwong [1] [2] [3] cubic equation of state for a pure compound. Subclasses
GCEOS, which provides the methods for solving the EOS and calculating its assorted relevant thermodynamic properties. Solves the EOS on initialization.Two of T, P, and V are needed to solve the EOS.
- Parameters
Notes
omega is allowed as an input for compatibility with the other EOS forms, but is not used.
References
- 1
Redlich, Otto., and J. N. S. Kwong. “On the Thermodynamics of Solutions. V. An Equation of State. Fugacities of Gaseous Solutions.” Chemical Reviews 44, no. 1 (February 1, 1949): 233-44. doi:10.1021/cr60137a013.
- 2
Poling, Bruce E. The Properties of Gases and Liquids. 5th edition. New York: McGraw-Hill Professional, 2000.
- 3
Walas, Stanley M. Phase Equilibria in Chemical Engineering. Butterworth-Heinemann, 1985.
Examples
>>> eos = RK(Tc=507.6, Pc=3025000, T=299., P=1E6) >>> eos.phase, eos.V_l, eos.H_dep_l, eos.S_dep_l ('l', 0.000151893468781, -26160.8424877, -63.013137852)
- Attributes
- omega
Methods
T_discriminant_zeros_analytical([valid])Method to calculate the temperatures which zero the discriminant function of the RK eos.
Method to calculate and its first and second derivatives for this EOS.
a_alpha_pure(T)Method to calculate for this EOS.
solve_T(P, V[, solution])Method to calculate T from a specified P and V for the RK EOS.
- T_discriminant_zeros_analytical(valid=False)[source]¶
Method to calculate the temperatures which zero the discriminant function of the RK eos. This is an analytical function with an 11-coefficient polynomial which is solved with numpy.
- Parameters
- validbool
Whether to filter the calculated temperatures so that they are all real, and positive only, [-]
- Returns
- T_discriminant_zeros
float Temperatures which make the discriminant zero, [K]
- T_discriminant_zeros
Notes
Calculated analytically. Derived as follows. Has multiple solutions.
>>> from sympy import * >>> P, T, V, R, b, a, Troot = symbols('P, T, V, R, b, a, Troot') >>> a_alpha = a/sqrt(T) >>> delta, epsilon = b, 0 >>> eta = b >>> B = b*P/(R*T) >>> deltas = delta*P/(R*T) >>> thetas = a_alpha*P/(R*T)**2 >>> epsilons = epsilon*(P/(R*T))**2 >>> etas = eta*P/(R*T) >>> a_coeff = 1 >>> b_coeff = (deltas - B - 1) >>> c = (thetas + epsilons - deltas*(B+1)) >>> d = -(epsilons*(B+1) + thetas*etas) >>> disc = b_coeff*b_coeff*c*c - 4*a_coeff*c*c*c - 4*b_coeff*b_coeff*b_coeff*d - 27*a_coeff*a_coeff*d*d + 18*a_coeff*b_coeff*c*d >>> new_disc = disc.subs(sqrt(T), Troot) >>> new_T_base = expand(expand(new_disc)*Troot**15) >>> ans = collect(new_T_base, Troot).args
- a_alpha_and_derivatives_pure(T)[source]¶
Method to calculate and its first and second derivatives for this EOS. Uses the set values of a.
- Parameters
- T
float Temperature at which to calculate the values, [-]
- T
- Returns
- c1 = 0.4274802335403414¶
Full value of the constant in the a parameter
- c2 = 0.08664034996495772¶
Full value of the constant in the b parameter
- solve_T(P, V, solution=None)[source]¶
Method to calculate T from a specified P and V for the RK EOS. Uses a, and b, obtained from the class’s namespace.
- Parameters
- Returns
- T
float Temperature, [K]
- T
Notes
The exact solution can be derived as follows; it is excluded for breviety.
>>> from sympy import * >>> P, T, V, R = symbols('P, T, V, R') >>> Tc, Pc = symbols('Tc, Pc') >>> a, b = symbols('a, b') >>> RK = Eq(P, R*T/(V-b) - a/sqrt(T)/(V*V + b*V)) >>> solve(RK, T)
Ideal Gas Equation of State¶
- class thermo.eos.IG(Tc=None, Pc=None, omega=None, T=None, P=None, V=None)[source]¶
Bases:
thermo.eos.GCEOSClass for solving the ideal gas equation in the GCEOS framework. This provides access to a number of derivatives and properties easily. It also keeps a common interface for all gas models. However, it is somewhat slow.
Subclasses
GCEOS, which provides the methods for solving the EOS and calculating its assorted relevant thermodynamic properties. Solves the EOS on initialization.Two of T, P, and V are needed to solve the EOS; values for Tc and Pc and omega, which are not used in the calculates, are set to those of methane by default to allow use without specifying them.
- Parameters
References
- 1
Smith, J. M, H. C Van Ness, and Michael M Abbott. Introduction to Chemical Engineering Thermodynamics. Boston: McGraw-Hill, 2005.
Examples
T-P initialization, and exploring each phase’s properties:
>>> eos = IG(T=400., P=1E6) >>> eos.V_g, eos.phase (0.003325785047261296, 'g') >>> eos.H_dep_g, eos.S_dep_g, eos.U_dep_g, eos.G_dep_g, eos.A_dep_g (0.0, 0.0, 0.0, 0.0, 0.0) >>> eos.beta_g, eos.kappa_g, eos.Cp_dep_g, eos.Cv_dep_g (0.0025, 1e-06, 0.0, 0.0) >>> eos.fugacity_g, eos.PIP_g, eos.Z_g, eos.dP_dT_g (1000000.0, 0.9999999999999999, 1.0, 2500.0)
Methods
Method to calculate and its first and second derivatives for this EOS.
a_alpha_pure(T)Method to calculate for the ideal gas law, which is zero.
solve_T(P, V[, solution])Method to calculate T from a specified P and V for the ideal gas equation of state.
volume_solutions(T, P[, b, delta, epsilon, ...])Calculate the ideal-gas molar volume in a format compatible with the other cubic EOS solvers.
- Zc = 1.0¶
float: Critical compressibility for an ideal gas is 1
- a = 0.0¶
float: a parameter for an ideal gas is 0
- a_alpha_and_derivatives_pure(T)[source]¶
Method to calculate and its first and second derivatives for this EOS. All values are zero.
- Parameters
- T
float Temperature at which to calculate the values, [-]
- T
- Returns
- b = 0.0¶
float: b parameter for an ideal gas is 0
- delta = 0.0¶
float: delta parameter for an ideal gas is 0
- epsilon = 0.0¶
float: epsilon parameter for an ideal gas is 0
- solve_T(P, V, solution=None)[source]¶
Method to calculate T from a specified P and V for the ideal gas equation of state.
- static volume_solutions(T, P, b=0.0, delta=0.0, epsilon=0.0, a_alpha=0.0)¶
Calculate the ideal-gas molar volume in a format compatible with the other cubic EOS solvers. The ideal gas volume is the first element; and the secodn and third elements are zero. This is implemented to allow the ideal-gas model to be compatible with the cubic models, whose equations do not work with parameters of zero.
- Parameters
- T
float Temperature, [K]
- P
float Pressure, [Pa]
- b
float,optional Coefficient calculated by EOS-specific method, [m^3/mol]
- delta
float,optional Coefficient calculated by EOS-specific method, [m^3/mol]
- epsilon
float,optional Coefficient calculated by EOS-specific method, [m^6/mol^2]
- a_alpha
float,optional Coefficient calculated by EOS-specific method, [J^2/mol^2/Pa]
- T
- Returns
Examples
>>> volume_solutions_ideal(T=300, P=1e7) (0.0002494338785445972, 0.0, 0.0)
Lists of Equations of State¶
- thermo.eos.eos_list = [<class 'thermo.eos.IG'>, <class 'thermo.eos.PR'>, <class 'thermo.eos.PR78'>, <class 'thermo.eos.PRSV'>, <class 'thermo.eos.PRSV2'>, <class 'thermo.eos.VDW'>, <class 'thermo.eos.RK'>, <class 'thermo.eos.SRK'>, <class 'thermo.eos.APISRK'>, <class 'thermo.eos.TWUPR'>, <class 'thermo.eos.TWUSRK'>, <class 'thermo.eos.PRTranslatedPPJP'>, <class 'thermo.eos.SRKTranslatedPPJP'>, <class 'thermo.eos.MSRKTranslated'>, <class 'thermo.eos.PRTranslatedConsistent'>, <class 'thermo.eos.SRKTranslatedConsistent'>]¶
list : List of all cubic equation of state classes.
- thermo.eos.eos_2P_list = [<class 'thermo.eos.PR'>, <class 'thermo.eos.PR78'>, <class 'thermo.eos.PRSV'>, <class 'thermo.eos.PRSV2'>, <class 'thermo.eos.VDW'>, <class 'thermo.eos.RK'>, <class 'thermo.eos.SRK'>, <class 'thermo.eos.APISRK'>, <class 'thermo.eos.TWUPR'>, <class 'thermo.eos.TWUSRK'>, <class 'thermo.eos.PRTranslatedPPJP'>, <class 'thermo.eos.SRKTranslatedPPJP'>, <class 'thermo.eos.MSRKTranslated'>, <class 'thermo.eos.PRTranslatedConsistent'>, <class 'thermo.eos.SRKTranslatedConsistent'>]¶
list : List of all cubic equation of state classes that can represent multiple phases.
Demonstrations of Concepts¶
Maximum Pressure at Constant Volume¶
Some equations of state show this behavior. At a liquid volume, if the temperature is increased, the pressure should increase as well to create that same volume. However in some cases this is not the case as can be demonstrated for this hypothetical dodecane-like fluid:
(Source code, png, hires.png, pdf)
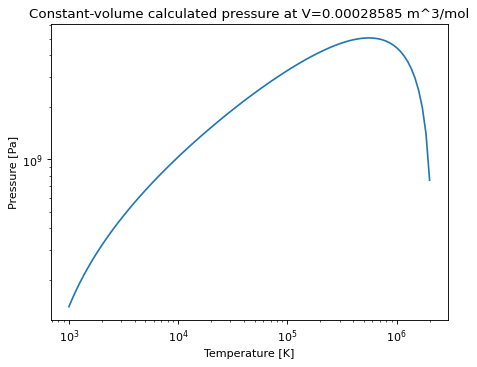
Through experience, it is observed that this behavior is only shown for some sets of critical constants. It was found that if the expression for is set to zero, an analytical expression can be determined for exactly what that maximum pressure is. Some EOSs implement this function as P_max_at_V; those that don’t, and fluids where there is no maximum pressure, will have that method but it will return None.
Debug Plots to Understand EOSs¶
The GCEOS.volume_errors method shows the relative error in the volume
solution. mpmath is requried for this functionality. It is not likely there
is an error here but many problems have been found in the past.
(Source code, png, hires.png, pdf)
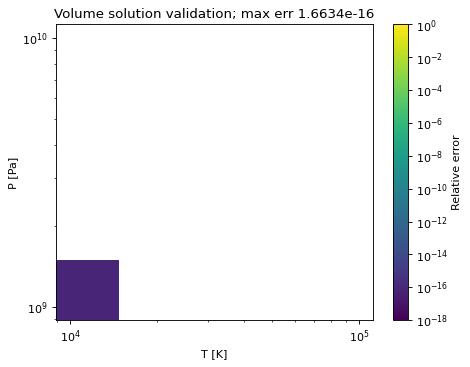
The GCEOS.PT_surface_special method shows some of the special curves of
the EOS.
(Source code, png, hires.png, pdf)
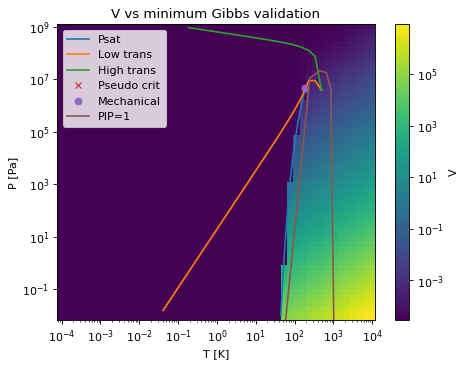
The GCEOS.a_alpha_plot method shows the alpha function curve. The
following sample shows the SRK’s default alpha function for methane.
(Source code, png, hires.png, pdf)
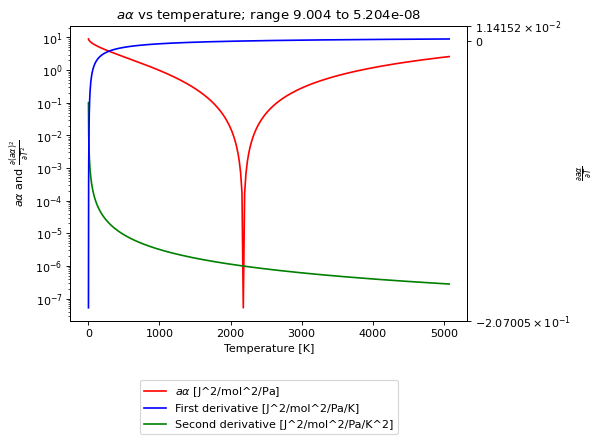
If this doesn’t look healthy, that is because it is not. There are strict thermodynamic consistency requirements that we know of today:
The alpha function must be positive and continuous
The first derivative must be negative and continuous
The second derivative must be positive and continuous
The third derivative must be negative
The first criterial and second criteria fail here.
There are two methods to review the saturation properties solution. The more general way is to review saturation properties as a plot:
(Source code, png, hires.png, pdf)
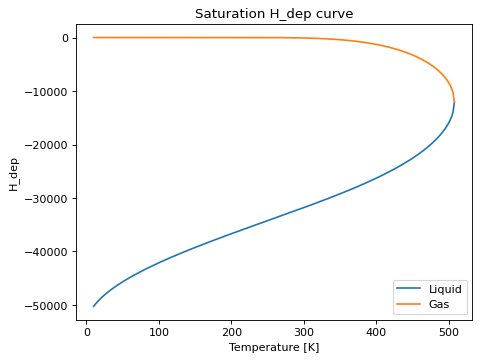
(Source code, png, hires.png, pdf)
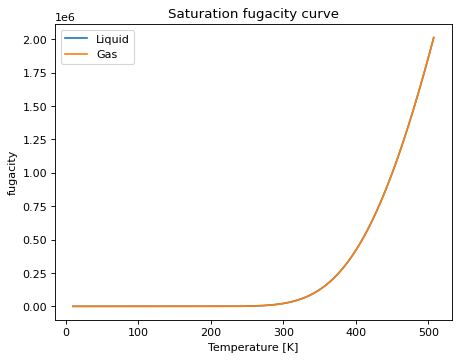
The second plot is more detailed, and is focused on the direct calculation of vapor pressure without using an iterative solution. It shows the relative error of the fit, which normally way below where it would present any issue - only 10-100x more error than it is possible to get with floating point numbers at all.
(Source code, png, hires.png, pdf)